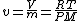- Applications
- Application examples
- Table of common specific volumes
- References
In thermodynamics, the specific volume of a substance is the ratio of the substance's volume to its mass. It is the reciprocal of density and an intrinsic property of matter as well. Specific volume is defined as the number of cubic meters occupied by one kilogram of a particular substance. The standard unit is the cubic meter per kilogram  .
.
Specific volume for an ideal gas is also equal to the gas constant (R) multiplied by the temperature and then divided by the pressure multiplied by molar mass of that ideal gas.
Since  and
and 
Typically, the specific volume of a substance is expressed in terms such as  ,
,  ,
,  , or
, or  .[1]
.[1]
Applications
Specific volume is commonly applied to:
- Molar volume
- Volume (thermodynamics)
- Partial molar volume
Imagine a variable-volume, airtight chamber containing a certain number of atoms of oxygen gas. Consider the following four examples:
- If the chamber is made smaller without allowing gas in or out, the density increases and the specific volume decreases.
- If the chamber expands without letting gas in or out, the density decreases and the specific volume increases.
- If the size of the chamber remains constant and new atoms of gas are injected, the density increases and the specific volume decreases.
- If the size of the chamber remains constant and some atoms are removed, the density decreases and the specific volume increases.
Specific volume is a property of materials, defined as the number of cubic meters occupied by one kilogram of a particular substance. The standard unit is the meter cubed per kilogram (m3/kg or m3·kg−1).
Sometimes specific volume is expressed in terms of the number of cubic centimeters occupied by one gram of a substance. In this case, the unit is the centimeter cubed per gram (cm3/g or cm3·g−1). To convert m3/kg to cm3/g, multiply by 1000; conversely, multiply by 0.001.
Specific volume is inversely proportional to density. If the density of a substance doubles, its specific volume, as expressed in the same base units, is cut in half. If the density drops to 1/10 its former value, the specific volume, as expressed in the same base units, increases by a factor of 10.
The density of gases changes with even slight variations in temperature, while densities of liquid and solids, which are generally thought of as incompressible, will change very little. Specific volume is the inverse of the density of a substance; therefore, careful consideration must be taken account when dealing with situations that involve gases. Small changes in temperature will have a noticeable effect on specific volumes.
The chart below is a visual representation of the relationship between specific volume and temperature. As stated above, specific volume varies noticeably with changes in temperature while the gas phase.
The average density of human blood is 1060 kg/m3. The specific volume that correlates to that density is 0.00094 m3/kg. Notice that the average specific volume of blood is almost identical to that of water: 0.00100 m3/kg.[2]
Application examples
If one sets out to determine the specific volume of an ideal gas, such as super heated steam, using the equation

where pressure is 2500 lbf/in2, R is 0.596, temperature is 1960 Rankine. In that case, the specific volume would equal 0.4672 in3/lb. However, if the temperature is changed to 1160 Rankine, the specific volume of the super heated steam would have changed to 0.2765 in3/lb, which is a 59% overall change.
Knowing the specific volumes of two or more substances allows one to find useful information for certain applications. For a substance X with a specific volume of 0.657 cm3/g and a substance Y with a specific volume 0.374 cm3/g, the density of each substance can be found by taking the inverse of the specific volume; therefore, substance X has a density of 1.522 g/cm3 and substance Y has a density of 2.673 g/cm3. With this information, the specific gravities of each substance relative to one another can be found. The specific gravity of substance X with respect to Y is 0.569, while the specific gravity of Y with respect to X is 1.756. Therefore, substance X will not sink if placed on Y.[3]
Table of common specific volumes
The table below displays densities and specific volumes for various common substances that may be useful. The values were recorded at standard temperature and pressure, which is defined as air at 0 °C (273.15 K, 32 °F) and 1 atm (101.325 kN/m2, 101.325 kPa, 14.7 psia, 0 psig, 30 in Hg, 760 torr).[4]
| Substance name | Density | Specific volume |
|---|---|---|
| (kg/m3) | (m3/kg) | |
| Air | 1.225 | 0.78 |
| Ice | 916.7 | 0.00109 |
| Water (liquid) | 1000 | 0.00100 |
| Salt Water | 1030 | 0.00097 |
| Mercury | 13546 | 0.00007 |
| R-22* | 3.66 | 0.273 |
| Ammonia | 0.769 | 1.30 |
| Carbon dioxide | 1.977 | 0.506 |
| Chlorine | 2.994 | 0.334 |
| Hydrogen | 0.0899 | 11.12 |
| Methane | 0.717 | 1.39 |
| Nitrogen | 1.25 | 0.799 |
| Steam* | 0.804 | 1.24 |
- values not taken at standard temperature and pressure
References
2. ^{{cite book|last=Silverthorn|first=Dee|title=Human Physiology|publisher=Pearson|isbn=978-0-321-55980-7}}
3. ^{{cite book|last=Walker|first=Jearl|title=Fundamentals of Physics|publisher=Halliday|isbn=978-0-470-04472-8}}
4. ^{{cite web|title=Engineering Tool Box|url=http://www.engineeringtoolbox.com/gas-density-d_158.html|accessdate=April 14, 2013}}
- Lupșa River (disambiguation)
- Lupșeni
- Luqa al-Injili
- Luqa (crater)
- Luqa International Airport
- Luq District
- Lu Qi (actor)
- Luqian Port Station
- Luqiao
- Luqiao Air Base
- Luqiao Airport
- Lu Qin
- Lu Qinzhai
- Luqiu Luwei
- Luqmān
- Luquan City
- Luquan, Hebei
- Luque, Paraguay
- Luquetia
- Luquetia abchasiella
- Luquetia lobella
- Luquetia orientella
- Luquillo Mountain babyboot orchid
- Luquillo Mountain stopper
- Lurabi