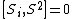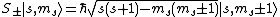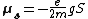- Derivation
- Algebra
- History
- Detection of spin The Stern–Gerlach experiment
- Energy levels from the Dirac equation
- Total spin of an atom or molecule
- See also
- References
- External links
}}
In atomic physics, the spin quantum number is a quantum number that parameterizes the intrinsic angular momentum (or spin angular momentum, or simply spin) of a given particle. The spin quantum number is the fourth of a set of quantum numbers (the principal quantum number, the azimuthal quantum number, the magnetic quantum number, and the spin quantum number), which completely describe the quantum state of an electron. It is designated by the letter {{mvar|s}}. It describes the energy, shape and orientation of orbitals. The name comes from a physical spinning (denoted by the letter s) about an axis that was proposed by Uhlenbeck and Goudsmit. However this simplistic picture was quickly realized to be physically impossible[1] and replaced by a more abstract quantum-mechanical description.
Derivation
As a solution for a certain partial differential equation, the quantized angular momentum (see angular momentum quantum number) can be written as:
where
is the quantized spin vector
is the norm of the spin vector
is the spin quantum number associated with the spin angular momentum
is the reduced Planck constant.
Given an arbitrary direction z (usually determined by an external magnetic field) the spin z-projection is given by
where {{mvar|m}}{{mvar|s}} is the secondary spin quantum number, ranging from −{{mvar|s}} to +{{mvar|s}} in steps of one. This generates {{math|1=2 {{mvar|s}} + 1}} different values of {{mvar|m}}{{mvar|s}}.
The allowed values for s are non-negative integers or half-integers. Fermions (such as the electron, proton or neutron) have half-integer values, whereas bosons (e.g., photon, mesons) have integer spin values.
Algebra
The algebraic theory of spin is a carbon copy of the angular momentum in quantum mechanics theory.
First of all, spin satisfies the fundamental commutation relation:
,
where εlmn is the (antisymmetric) Levi-Civita symbol. This means that it is impossible to know two coordinates of the spin at the same time because of the restriction of the uncertainty principle.
Next, the eigenvectors of  and
and  satisfy:
satisfy:
where  are the creation and annihilation (or "raising" and "lowering" or "up" and "down") operators.
are the creation and annihilation (or "raising" and "lowering" or "up" and "down") operators.
History
Early attempts to explain the behavior of electrons in atoms focused on solving the Schrödinger wave equation for the hydrogen atom, the simplest possible case, with a single electron bound to the atomic nucleus. This was successful in explaining many features of atomic spectra.
The solutions required each possible state of the electron to be described by three "quantum numbers". These were identified as, respectively, the electron "shell" number {{mvar|n}}, the "orbital" number {{mvar|l}}, and the "orbital angular momentum" number {{mvar|m}}. Angular momentum is a so-called "classical" concept measuring the momentum{{citation needed|date=February 2012}} of a mass in circular motion about a point. The shell numbers start at 1 and increase indefinitely. Each shell of number {{mvar|n}} contains {{mvar|n}}² orbitals. Each orbital is characterized by its number {{mvar|l}}, where {{mvar|l}} takes integer values from 0 to {{mvar|n}}−1, and its angular momentum number {{mvar|m}}, where {{mvar|m}} takes integer values from +{{mvar|l}} to −{{mvar|l}}. By means of a variety of approximations and extensions, physicists were able to extend their work on hydrogen to more complex atoms containing many electrons.
Atomic spectra measure radiation absorbed or emitted by electrons "jumping" from one "state" to another, where a state is represented by values of {{mvar|n}}, {{mvar|l}}, and {{mvar|m}}. The so-called "Transition rule" limits what "jumps" are possible. In general, a jump or "transition" is allowed only if all three numbers change in the process. This is because a transition will be able to cause the emission or absorption of electromagnetic radiation only if it involves a change in the electromagnetic dipole of the atom.
However, it was recognized in the early years of quantum mechanics that atomic spectra measured in an external magnetic field (see Zeeman effect) cannot be predicted with just {{mvar|n}}, {{mvar|l}}, and {{mvar|m}}.
In January 1925, when Ralph Kronig was still a Columbia University PhD student, he first proposed electron spin after hearing Wolfgang Pauli in Tübingen. Werner Heisenberg and Pauli immediately hated the idea. They had just ruled out all imaginable actions from quantum mechanics. Now Kronig was proposing to set the electron rotating in space. Pauli especially ridiculed the idea of spin, saying that "it is indeed very clever but of course has nothing to do with reality". Faced with such criticism, Kronig decided not to publish his theory and the idea of electron spin had to wait for others to take the credit.[2] Ralph Kronig had come up with the idea of electron spin several months before George Uhlenbeck and Samuel Goudsmit. Most textbooks credit these two Dutch physicists with the discovery.
Pauli subsequently proposed (also in 1925) a new quantum degree of freedom (or quantum number) with two possible values, in order to resolve inconsistencies between observed molecular spectra and the developing theory of quantum mechanics.
Shortly thereafter Uhlenbeck and Goudsmit identified Pauli's new degree of freedom as electron spin.
==Electron spin==
The spin angular momentum is characterized by a quantum number; s = 1/2 specifically for electrons. In a way analogous to other quantized angular momenta, L, it is possible to obtain an expression for the total spin angular momentum:
where
is the reduced Planck constant.
The hydrogen spectra fine structure is observed as a doublet corresponding to two possibilities for the z-component of the angular momentum, where for any given direction z:
whose solution has only two possible z-components for the electron. In the electron, the two different spin orientations are sometimes called "spin-up" or "spin-down".
The spin property of an electron would give rise to magnetic moment, which was a requisite for the fourth quantum number. The electron spin magnetic moment is given by the formula:
where
{{mvar|e}} is the charge of the electron
{{mvar|g}} is the Landé g-factor
and by the equation:
where  is the Bohr magneton.
is the Bohr magneton.
When atoms have even numbers of electrons the spin of each electron in each orbital has opposing orientation to that of its immediate neighbor(s). However, many atoms have an odd number of electrons or an arrangement of electrons in which there is an unequal number of "spin-up" and "spin-down" orientations. These atoms or electrons are said to have unpaired spins that are detected in electron spin resonance.
Detection of spin
When lines of the hydrogen spectrum are examined at very high resolution, they are found to be closely spaced doublets. This splitting is called fine structure, and was one of the first experimental evidences for electron spin. The direct observation of the electron's intrinsic angular momentum was achieved in the Stern–Gerlach experiment.
The Stern–Gerlach experiment
The theory of spatial quantization of the spin moment of the momentum of electrons of atoms situated in the magnetic field needed to be proved experimentally. In 1920 (two years before the theoretical description of the spin was created) Otto Stern and Walter Gerlach observed it in the experiment they conducted.
Silver atoms were evaporated using an electric furnace in a vacuum. Using thin slits, the atoms were guided into a flat beam and the beam sent through an in-homogeneous magnetic field before colliding with a metallic plate. The laws of classical physics predict that the collection of condensed silver atoms on the plate should form a thin solid line in the same shape as the original beam. However, the in-homogeneous magnetic field caused the beam to split in two separate directions, creating two lines on the metallic plate.
The phenomenon can be explained with the spatial quantization of the spin moment of momentum. In atoms the electrons are paired such that one spins upward and one downward, neutralizing the effect of their spin on the action of the atom as a whole. But in the valence shell of silver atoms, there is a single electron whose spin remains unbalanced.
The unbalanced spin creates spin magnetic moment, making the electron act like a very small magnet. As the atoms pass through the in-homogeneous magnetic field, the force moment in the magnetic field influences the electron's dipole until its position matches the direction of the stronger field. The atom would then be pulled toward or away from the stronger magnetic field a specific amount, depending on the value of the valence electron's spin. When the spin of the electron is +1/2 the atom moves away from the stronger field, and when the spin is −1/2 the atom moves toward it. Thus the beam of silver atoms is split while traveling through the in-homogeneous magnetic field, according to the spin of each atom's valence electron.
In 1927 Phipps and Taylor conducted a similar experiment, using atoms of hydrogen with similar results. Later scientists conducted experiments using other atoms that have only one electron in their valence shell: (copper, gold, sodium, potassium). Every time there were two lines formed on the metallic plate.
The atomic nucleus also may have spin, but protons and neutrons are much heavier than electrons (about 1836 times), and the magnetic dipole moment is inversely proportional to the mass. So the nuclear magnetic dipole momentum is much smaller than that of the whole atom. This small magnetic dipole was later measured by Stern, Frisch and Easterman.
Energy levels from the Dirac equation
In 1928, Paul Dirac developed a relativistic wave equation, now termed the Dirac equation, which predicted the spin magnetic moment correctly, and at the same time treated the electron as a point-like particle. Solving the Dirac equation for the energy levels of an electron in the hydrogen atom, all four quantum numbers including {{mvar|s}} occurred naturally and agreed well with experiment.
Total spin of an atom or molecule
For some atoms the spins of several unpaired electrons (s1, s2, ...) are coupled to form a total spin quantum number S.[3][4] This occurs especially in light atoms (or in molecules formed only of light atoms) when spin-orbit coupling is weak compared to the coupling between spins or the coupling between orbital angular momenta, a situation known as LS coupling because L and S are constants of motion. Here L is the total orbital angular momentum quantum number.[4]
For atoms with a well-defined S, the multiplicity of a state is defined as (2S+1). This is equal to the number of different possible values of the total (orbital plus spin) angular momentum J for a given (L, S) combination, provided that S ≤ L (the typical case). For example, if S = 1, there are three states which form a triplet. The eigenvalues of Sz for these three states are +1ħ, 0 and -1ħ.[3] The term symbol of an atomic state indicates its values of L, S, and J.
See also
- Total angular momentum quantum number
- Rotational spectroscopy
- Basic quantum mechanics
References
2. ^{{cite book|last=Bertolotti|first=Mario|title=The History of the Laser|publisher=CRC Press|volume=|issue=|pages=150–153|doi=|year=2004|url=https://books.google.co.uk/books?id=JObDnEtzMJUC&source=gbs_navlinks_s|accessdate=22 March 2017}}
3. ^1 Merzbacher E., Quantum Mechanics (3rd ed., John Wiley 1998) p.430-1 {{ISBN|0-471-88702-1}}
4. ^1 Atkins P. and de Paula J. Physical Chemistry (8th ed., W.H.Freeman 2006), p.352 {{ISBN|0-7167-8759-8}}
External links
- Full treatment of Spin--including origins, evolution of Spin Theory, and details of the Spin equations
- Stars in Your Eyes
- Stars in Your Eyes (Booster Gold)
- Star Sirius
- Starsis
- Star Sisters
- Stars Kill Rock
- Starsky
- Starsky and Hutch on Playboy Island
- Starsky and Hutch (pilot)
- Starsky Wong
- Starslayer: The Log of the Jolly Roger
- Starslip Crisis
- Stars (Makai album)
- Stars(Mario)
- Stars Medley (Stars on 45 album)
- Stars members
- STARS members in Resident Evil
- S.T.A.R.S. Members (Resident Evil)
- Stars (mini-album)
- Starsmith
- Stars nearest to Earth
- Stars (newspaper)
- Star snot
- Stars'n'stripes
- Star Soccer