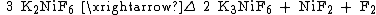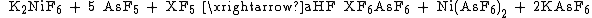- References
| ImageFile = Mg2FeH6 from X-ray.jpg
| ImageSize =
| IUPACName = potassium hexafluoronickelate(IV)
| OtherNames =
|Section1={{Chembox Identifiers
| CASNo = 17218-47-2
| CASNo_Ref = {{cascite|correct|scifinder}}
| PubChem =
| ChemSpiderID = 21241633
| SMILES = [K+].[K+].F[Ni-2](F)(F)(F)(F)F
| InChI = InChI=1S/6FH.2K.Ni/h6*1H;;;/q;;;;;;2*+1;+4/p-6
|Section2={{Chembox Properties
| Formula = K2NiF6
| MolarMass = 250.880
| Appearance =
| Density =
| MeltingPt =
| BoilingPt =
| Solubility =
|Section3={{Chembox Hazards
| ExternalSDS = [https://www.americanelements.com/printpdf/potassium-hexafluoronickelate-iv-17218-47-2/sds External SDS]
| GHSPictograms = {{GHS06}}{{GHS07}}{{GHS08}}[1]
| GHSSignalWord = Danger[1]
| HPhrases = {{H-phrases|302|312|317|331|350}}[1]
| PPhrases = {{P-phrases|201|261|280|304+340|405|501}}[1]
| MainHazards =
| FlashPt =
| AutoignitionPt =
}}
Potassium hexafluoronickelate(IV) is an inorganic compound with the chemical formula {{chem|K|2|NiF|6}}. It can be produced through the reaction of potassium fluoride, nickel dichloride, and fluorine.
It reacts violently with water, releasing oxygen. It dissolves in anhydrous hydrogen fluoride to produce a light-red solution. Potassium hexafluoronickelate(IV) decomposes at 350 °C, forming potassium hexafluoronickelate(III), nickel(II) fluoride, and fluorine:[2]{{better source|date=December 2013}}
Potassium hexafluoronickelate is a strong oxidant. It can turn chlorine pentafluoride and bromine pentafluoride into {{chem|ClF|6|+}} and {{chem|BrF|6|+}}, respectively:[3]
( X = Cl or Br , -60 °C , aHF = anhydrous hydrogen fluoride).
It adopts the structure seen for K2PtCl6.[4]
References
2. ^{{zh-hans}}{{cite book|title=《无机化学丛书》第九卷:锰分族、铁系、铂系|pages=P333|author=张青莲|isbn=7-03-002238-6|location=北京|publisher=科学出版社}}
3. ^{{cite journal|doi=10.1021/ic001024|title=Novel Synthesis of ClF6+ and BrF6+ Salts|year=2001|last1=Schroer|first1=Thorsten|last2=Christe|first2=Karl O.|journal=Inorganic Chemistry|volume=40|issue=10|pages=2415–9|pmid=11327921}}
4. ^Taylor, J. C. "A comparison of profile decomposition and Rietveld methods for structurtal refinement with powder diffraction data" Zeitschrift für Kristallographie 1987, volume 181, p151-160.
- Emmanuel Okah
- Emmanuel Okine
- Emmanuel Okocha
- Emmanuel Okoye
- Emmanuel Okwi
- Emmanuel Okwudili
- Emmanuel Okyere Boateng
- Emmanuel Onwe
- Emmanuel Oshoffa
- Emmanuel, Oswaldtwistle
- Emmanuel Otteh
- Emmanuel Pauwels
- Emmanuel Perea
- Emmanuel Peristerakis
- Emmanuel Persillier-Lachapelle
- Emmanuel Philibert Amadeus of Savoy
- Emmanuel Philibert Amadeus of Savoy-Carignano, Prince of Carignano
- Emmanuel Philibert Amadeus of Savoy, Prince of Carignano
- Emmanuel-Philibert de Savoie (1528-1580)
- Emmanuel Philibert of Savoy, Prince of Carignano
- Emmanuel Philibert, Prince of Carignan
- Emmanuel Pierre de Bethune
- Emmanuel Pierre Ghislain de Bethune
- Emmanuel Pierre Marie Ghislain de Bethune
- Emmanuel Piget