- Overview
- Theoretical model
- Reciprocal Activation Energy
- References
In chemical kinetics, the Aquilanti–Mundim deformed Arrhenius model is a generalization of the standard Arrhenius law.
Overview
Arrhenius plots, which are used to represent the effects of temperature on the rates of chemical and biophysical processes and on various transport phenomena in materials science, may exhibit deviations from linearity. Account of curvature is provided here by a formula, which involves a deformation of the exponential function, of the kind recently encountered in treatments of non-extensivity in statistical mechanics.
Theoretical model
Svante Arrhenius (1889) model are often used to characterize the effect of temperature on the rates of chemical reactions.[1] The Arrhenius equation gave a simple and powerful law, which in a vast generality of cases describes the dependence on absolute temperature 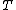 of the rate constant as following,
of the rate constant as following,
 (1)
(1)
where 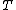 is the absolute temperature,
is the absolute temperature,  is the gas constant and the factor
is the gas constant and the factor  varies only slightly with temperature. The meaning attached to the energy of activation
varies only slightly with temperature. The meaning attached to the energy of activation 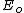 is as the minimum energy, which molecules need have to overcome the threshold to reaction. Therefore, the year 1889 can be considered as the birth date of reactive dynamics as the study of the motion of atoms and molecules in a reactive event. Eq. (1) was motivated by the 1884 discovery by van't Hoff [2] of the exponential dependence from the temperature of the equilibrium constants for most reactions: Eq.(1), when used for both a reaction and its inverse, agrees with van't Hoff's equation interpreting chemical equilibrium as dynamical at the microscopic level. In case of a single rate-limited thermally activated process, an Arrhenius plot gives a straight line, from which the activation energy and the pre-exponential factor can both be determined.
is as the minimum energy, which molecules need have to overcome the threshold to reaction. Therefore, the year 1889 can be considered as the birth date of reactive dynamics as the study of the motion of atoms and molecules in a reactive event. Eq. (1) was motivated by the 1884 discovery by van't Hoff [2] of the exponential dependence from the temperature of the equilibrium constants for most reactions: Eq.(1), when used for both a reaction and its inverse, agrees with van't Hoff's equation interpreting chemical equilibrium as dynamical at the microscopic level. In case of a single rate-limited thermally activated process, an Arrhenius plot gives a straight line, from which the activation energy and the pre-exponential factor can both be determined.
However, advances in experimental and theoretical methods have revealed the existence of deviation from Arrhenius behavior (Fig.1).
To overcome this problem, Aquilanti and Mundim[3] proposed (2010) a generalized Arrhenius law based on algebraic deformation of the usual exponential function. Starting from the Euler[4] exponential definition given by,
 (2)
(2)
defining the deformed exponential function as,
 (3)
(3)
Identifying the deformation parameter  as a continuous generalization of
as a continuous generalization of 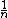 . At the limit
. At the limit  the d-exponential function,
the d-exponential function,  , coincides with the usual exponential according to the well-known limit due to Euler, that is,
, coincides with the usual exponential according to the well-known limit due to Euler, that is,
 (4)
(4)
This definition was first used in thermodynamics and statistical mechanics by Landau.[5] In the most recent scientific literature, there is a variety of deformed algebras with applications in different areas of science.[6][7] Considering the d-exponential function, we introduce the deformed reaction rate coefficient,  , in the following way,
, in the following way,
 (5)
(5)
and at the limit  the usual Arrhenius reaction law is recovered (Figs.1 and 1a).
the usual Arrhenius reaction law is recovered (Figs.1 and 1a).  is pre-exponential factor. Taking the logarithm of
is pre-exponential factor. Taking the logarithm of  , Eq.(5), we obtain the following expression for the non-Arrhenius plot,
, Eq.(5), we obtain the following expression for the non-Arrhenius plot,
 (6)
(6)
The logarithm of the reaction rate coefficient against reciprocal temperature shows a curvature, rather than the straight-line behavior described by the usual Arrhenius law (Figs.1 and 1a).
In Tolman’s[8] definition the barrier or activation energy is a phenomenological quantity defined in terms of the slope of an Arrhenius law; it is usually assumed to be independent of absolute temperature (T), requires only local equilibrium and in general is given by
 (7)
(7)
where  is constant and
is constant and  is the ideal gas constant.
is the ideal gas constant.
To generalize Tolman´s definition, in the case chemical reactions, we assume that the barrier or activation energy is a function of the temperature given by the following differential equation,
 (8)
(8)
where  (constant) at limit
(constant) at limit  and the usual activation energy law is recovered as a constant. Noticeably, on the contrary of the usual Arrhenius case, the barrier or activation energy is temperature dependent and
and the usual activation energy law is recovered as a constant. Noticeably, on the contrary of the usual Arrhenius case, the barrier or activation energy is temperature dependent and  has different concavities depending on the value of the d parameter (see Figs.1 and 1a). Thus, a positive convexity means that
has different concavities depending on the value of the d parameter (see Figs.1 and 1a). Thus, a positive convexity means that  decreases with increasing temperature. This general result is explained by a new Tolman interpretation of the activation energy through Eq.(8).
decreases with increasing temperature. This general result is explained by a new Tolman interpretation of the activation energy through Eq.(8).
In the recent literature, it is possible find different applications to verify the applicability of this new chemical reaction formalism[9]
[10][11][12][13][14][15][16][17][18]Reciprocal Activation Energy
 can be considered as temperature dependent. It was postulated as the basic expansion the reciprocal-activation reciprocal-temperature relationship, for which can provide a formal mathematical justification by Tolman Theorem. Tolman’s function when written as the logarithmic derivative of the rate constants with respect to
can be considered as temperature dependent. It was postulated as the basic expansion the reciprocal-activation reciprocal-temperature relationship, for which can provide a formal mathematical justification by Tolman Theorem. Tolman’s function when written as the logarithmic derivative of the rate constants with respect to 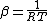 , Eq. (7), the concept to an activation energy represents an energetic obstacle to the progress of the reaction: therefore its reciprocal can be interpreted as a measure of the propensity for the reaction to proceed and defined as the specific transitivity (
, Eq. (7), the concept to an activation energy represents an energetic obstacle to the progress of the reaction: therefore its reciprocal can be interpreted as a measure of the propensity for the reaction to proceed and defined as the specific transitivity ( ) of the process:
) of the process:
 (9)
(9)
This notation emphasizes the fact that in general the transitivity can take a gamma of values, but not including abrupt changes e.g. in the mechanism or in the phases of reactants. If it is admit a Laurent expansion in a neighbourhood around a reference value, it is possible recover the Eqs. (6) and (8).[19]
What it is call the sub-Arrhenius behaviour would be accounted for traditionally by introducing a tunnelling parameter ( ) in the conventional Transition-State-Theory. In the
) in the conventional Transition-State-Theory. In the  -TST formulation, it is replace the factor
-TST formulation, it is replace the factor  in the TST rate constant by the deformed exponential function, Eq. (3), yielding:[18]
in the TST rate constant by the deformed exponential function, Eq. (3), yielding:[18]
 (10)
(10)
where  is Planck constant,
is Planck constant,  is Boltzmann constant and
is Boltzmann constant and 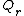 is the (translational, vibrational and rotational) partition functions of the reactants, and
is the (translational, vibrational and rotational) partition functions of the reactants, and  is the partition function of the activated complex. In Ref.,[11] the significance of the parameter
is the partition function of the activated complex. In Ref.,[11] the significance of the parameter  and an explicit procedure for its calculation were proposed, which it is inversely proportional to the square of the barrier height (
and an explicit procedure for its calculation were proposed, which it is inversely proportional to the square of the barrier height (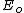 )and directly proportional to the square of the frequency for crossing the barrier (
)and directly proportional to the square of the frequency for crossing the barrier ( ) at a saddle point in the potential energy surface:
) at a saddle point in the potential energy surface:
 (11)
(11)
References
2. ^J. H. van't Hoff, 1884 in his book Études de Dynamique chimique.
3. ^V. Aquilanti, K. C. Mundim, M. Elango, S. Kleijn, T. Kasai, Chem. Phys. Lett. 2010, 498, 209.
4. ^L. Euler, Introductio in analysin infinitorum, Lugduni: Apud Bernuset, Delamolliere, Falque & soc., Editio nova., 1797.
5. ^E. M. Landau, L. D.; Lifshitz, Statistal Physics, Elsevier Butterworth-Heinemann, 1951.
6. ^E. P. Borges, Phys. A Stat. Mech. its Appl., 2004, 340, 95–101.
7. ^C. Tsallis, J. Stat. Phys., 1988, 52, 479–487.
8. ^R. C. Tolman, J. Am. Chem. Soc. 1920, 42, 2506.
9. ^L. Agreda, N.J. J Therm Anal Calorim. 2016 126: 1175. doi:10.1007/s10973-016-5566-8.
10. ^S. Rampino and Y. V. Suleimanov, J. Phys. Chem. A, 2016, 120 (50), pp 9887–9893.
11. ^1 V. H. C. Silva, V. Aquilanti, H. C. B. de Oliveira, K. C. Mundim, Chem. Phys. Lett. 2013, 590, 201.
12. ^S. Cavalli, V. Aquilanti, K. C. Mundim, D. De Fazio, J. Phys. Chem. A 2014, 118, 6632.
13. ^N. D. Coutinho, V. H. C. Silva, H. C. B. de Oliveira, A. J. Camargo, K. C. Mundim, V. Aquilanti, J. Phys. Chem. Lett. 2015, 6, 1553.
14. ^N. D. Coutinho, V. Aquilanti, V. H. C. Silva, A. J. Camargo, K. C. Mundim, H. C. B. de Oliveira, J. Phys. Chem. A 2016, 120, 5408.
15. ^V. Aquilanti, K. C. Mundim, S. Cavalli, D. De Fazio, A. Aguilar, J. M. Lucas, Chem. Phys. 2012, 398, 186.
16. ^K. C. Mundim and M. S. P. Mundim, Rev. Proc. Químicos. 2013, 14, 21.
17. ^V. H. C. Silva, H. C. B. Oliveira and K. C. Mundim, Rev. Proc. Químicos. 2013, 14, 9.
18. ^1 V. H. Carvalho-Silva, V. Aquilanti, H.C. de Oliveira, K. C. Mundim, J. Comput. Chem. 2017, 38, 178–188.
19. ^{{Cite journal|last=Aquilanti|first=Vincenzo|last2=Coutinho|first2=Nayara Dantas|last3=Carvalho-Silva|first3=Valter Henrique|date=2017-04-28|title=Kinetics of low-temperature transitions and a reaction rate theory from non-equilibrium distributions|url=http://rsta.royalsocietypublishing.org/content/375/2092/20160201|journal=Phil. Trans. R. Soc. A|language=en|volume=375|issue=2092|pages=20160201|doi=10.1098/rsta.2016.0201|issn=1364-503X|pmid=28320904|pmc=5360900}}
- Hoshonto
- Hosho Nyorai
- Hoshu
- Hoshu jugyoko
- Hoshu Jugyoko
- Hoshu-jugyo-ko
- Hoshu jugyo ko
- Hoshu Jugyo Ko
- HOSHU JYUGYO KO
- Hoshu Jyugyo Ko
- Hoshu jyugyo ko
- Hoshu Ko
- Hoshu-ko
- Hoshuko
- Hoshu-Ko
- Hoshuko Montreal
- Hoshuko Montréal
- Hoshukou
- Hoshu-Shogakko
- Hoshuu-jugyoko
- Hoshuu jugyou kou
- Hoshuukoo
- Hoshuukou
- Hoshuyama Station
- Hoshû jugyô kô