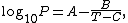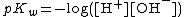- Liquid physical properties
- Water/steam equilibrium properties
- Melting point of ice at various pressures
- Table of various forms of ice
- Phase diagram
- Water with dissolved NaCl
- Self-ionization
- Self-diffusion coefficients
- Additional data translated from German "Wasser (Stoffdaten)" page Physical and thermodynamic tables Standard conditions Triple point Saturated vapor pressure Formulas
- Magnetic susceptibility
- References
- Bibliography
- External links
This page provides supplementary data to the article properties of water.
Further comprehensive authoritative data can be found at the NIST Webbook page on thermophysical properties of fluids. Except where noted otherwise, data relate to standard ambient temperature and pressure.
==Structure and properties==
| Structure and properties | |
|---|---|
| Index of refraction, nD | 1.333 at 20 °C |
| Dielectric constant[1] 88.00 at 0 °C 86.04 at 5 °C 84.11 at 10 °C 82.22 at 15 °C 80.36 at 20 °C 78.54 at 25 °C 76.75 at 30 °C 75.00 at 35 °C 73.28 at 40 °C 71.59 at 45 °C 69.94 at 50 °C 66.74 at 60 °C 63.68 at 70 °C 60.76 at 80 °C 57.98 at 90 °C 55.33 at 100 °C | |
| Bond strength | 492.215 kJ/mol O–H bond dissociation energy[2] |
| Bond length | 95.87 pm (equilibrium)[3] |
| Bond angle | 104.48° (equilibrium) [4][5] |
| Magnetic susceptibility | −9.04 × 10−6 volume SI units[6] |
==Thermodynamic properties==
| Phase behavior | |
|---|---|
| Triple point | 273.16 K (0.01 °C), 611.73 Pa |
| Critical point | 647 K (374 °C), 22.1 MPa |
| Enthalpy change of fusion at 273.15 K, ΔfusH | 6.01 kJ/mol |
| Entropy change of fusion at 273.15 K, 1 bar, ΔfusS | 22.0 J/(mol·K) |
| Std enthalpy change of vaporization, ΔvapH | 44.0 kJ/mol |
| Enthalpy change of vaporization at 373.15 K, ΔvapH | 40.68 kJ/mol |
| Std entropy change of vaporization, ΔvapS | 118.89 J/(mol·K) |
| Entropy change of vaporization at 373.15 K, ΔvapS | 109.02 J/(mol·K) |
| Enthalpy change of sublimation at 273.15 K, ΔsubH | 51.1 kJ/mol |
| Std entropy change of sublimation at 273.15 K, 1 bar, ΔsubS | ~144 J/(mol·K) |
| Molal freezing point constant | −1.858 °C kg/mol |
| Molal boiling point constant | 0.512 °C kg/mol |
| Solid properties | |
| Std enthalpy change of formation, ΔfH | −291.83 kJ/mol |
| Standard molar entropy, S | 41 J/(mol K) |
| Heat capacity, cp | 12.2 J/(mol K) at −200 °C 15.0 J/(mol K) at −180 °C 17.3 J/(mol K) at −160 °C 19.8 J/(mol K) at −140 °C 24.8 J/(mol K) at −100 °C 29.6 J/(mol K) at −60 °C 32.77 J/(mol K) at −38.3 °C 33.84 J/(mol K) at −30.6 °C 35.20 J/(mol K) at −20.8 °C 36.66 J/(mol K) at −11.0 °C 37.19 J/(mol K) at −4.9 °C 37.84 J/(mol K) at −2.2 °C |
| Liquid properties | |
| Std enthalpy change of formation, ΔfH | −285.83 kJ/mol |
| Standard molar entropy, S | 69.95 J/(mol K) |
| Heat capacity, cp | 75.97 J/(mol K) and 4.2176 J/(g·K) at 0 °C 75.42 J/(mol K) and 4.1921 J/(g·K) at 10 °C 75.33 J/(mol K) and 4.1818 J/(g·K) at 20 °C 75.28 J/(mol K) and 4.1814 J/(g·K) at 25 °C 75.26 J/(mol K) and 4.1784 J/(g·K) at 30 °C 75.26 J/(mol K) and 4.1785 J/(g·K) at 40 °C 75.30 J/(mol K) and 4.1806 J/(g·K) at 50 °C 75.37 J/(mol K) and 4.1843 J/(g·K) at 60 °C 75.46 J/(mol K) and 4.1895 J/(g·K) at 70 °C 75.58 J/(mol K) and 4.1963 J/(g·K) at 80 °C 75.74 J/(mol K) and 4.2050 J/(g·K) at 90 °C 75.94 J/(mol K) and 4.2159 J/(g·K) at 100 °C |
| Gas properties | |
| Std enthalpy change of formation, ΔfH | −241.83 kJ/mol |
| Standard molar entropy, S | 188.84 J/(mol K) |
| Heat capacity, cp | 36.5 J/(mol K) at 100 °C 36.1 J/(mol K) at 200 °C 36.2 J/(mol K) at 400 °C 37.9 J/(mol K) at 700 °C 41.4 J/(mol K) at 1000 °C |
| Heat capacity, cv | 27.5 J/(mol K) at 100 °C 27.6 J/(mol K) at 200 °C 27.8 J/(mol K) at 400 °C 29.5 J/(mol K) at 700 °C 33.1 J/(mol K) at 1000 °C |
| Heat capacity ratio, γ = cp/cv | 1.324 at 100 °C 1.310 at 200 °C 1.301 at 400 °C 1.282 at 700 °C 1.252 at 1000 °C |
| van der Waals' constants | a = 553.6 L2 kPa/mol2 b = 0.03049 L/mol |
Liquid physical properties
| Velocity of sound in water | |
|---|---|
| c in distilled water at 25 °C | 1498 m/s |
| c at other temperatures[7] | 1403 m/s at 0 °C 1427 m/s at 5 °C 1447 m/s at 10 °C 1481 m/s at 20 °C 1507 m/s at 30 °C 1526 m/s at 40 °C 1541 m/s at 50 °C 1552 m/s at 60 °C 1555 m/s at 70 °C 1555 m/s at 80 °C 1550 m/s at 90 °C 1543 m/s at 100 °C |
| Density[8][9] | |
| 0.983854 g/cm3 at −30 °C | 0.99221 g/cm3 at 40 °C |
| 0.993547 g/cm3 at −20 °C | 0.99022 g/cm3 at 45 °C |
| 0.998117 g/cm3 at −10 °C | 0.98804 g/cm3 at 50 °C |
| 0.9998395 g/cm3 at 0 °C | 0.98570 g/cm3 at 55 °C |
| 0.999972 g/cm3 at 3.984 °C[10] | |
| 0.9999720 g/cm3 at 4 °C | 0.98321 g/cm3 at 60 °C |
| 0.99996 g/cm3 at 5 °C | 0.98056 g/cm3 at 65 °C |
| 0.9997026 g/cm3 at 10 °C | 0.97778 g/cm3 at 70 °C |
| 0.9991026 g/cm3 at 15 °C | 0.97486 g/cm3 at 75 °C |
| 0.9982071 g/cm3 at 20 °C | 0.97180 g/cm3 at 80 °C |
| 0.9977735 g/cm3 at 22 °C | 0.96862 g/cm3 at 85 °C |
| 0.9970479 g/cm3 at 25 °C | 0.96531 g/cm3 at 90 °C |
| 0.9956502 g/cm3 at 30 °C | 0.96189 g/cm3 at 95 °C |
| 0.99403 g/cm3 at 35 °C | 0.95835 g/cm3 at 100 °C |
| The values below 0 °C refer to supercooled water. | |
| Viscosity[11] | |
| 1.7921 mPa·s (cP) at 0 °C | 0.5494 mPa·s at 50 °C |
| 1.5188 mPa·s at 5 °C | 0.5064 mPa·s at 55 °C |
| 1.3077 mPa·s at 10 °C | 0.4688 mPa·s at 60 °C |
| 1.1404 mPa·s at 15 °C | 0.4355 mPa·s at 65 °C |
| 1.0050 mPa·s at 20 °C | 0.4061 mPa·s at 70 °C |
| 0.8937 mPa·s at 25 °C | 0.3799 mPa·s at 75 °C |
| 0.8007 mPa·s at 30 °C | 0.3635 mPa·s at 80 °C |
| 0.7225 mPa·s at 35 °C | 0.3355 mPa·s at 85 °C |
| 0.6560 mPa·s at 40 °C | 0.3165 mPa·s at 90 °C |
| 0.5988 mPa·s at 45 °C | 0.2994 mPa·s at 95 °C |
| 0.2838 mPa·s at 100 °C | |
| Surface tension[12] | |
| 75.64 dyn/cm at 0 °C | 69.56 dyn/cm at 40 °C |
| 74.92 dyn/cm at 5 °C | 68.74 dyn/cm at 45 °C |
| 74.22 dyn/cm at 10 °C | 67.91 dyn/cm at 50 °C |
| 73.49 dyn/cm at 15 °C | 66.18 dyn/cm at 60 °C |
| 72.75 dyn/cm at 20 °C | 64.42 dyn/cm at 70 °C |
| 71.97 dyn/cm at 25 °C | 62.61 dyn/cm at 80 °C |
| 71.18 dyn/cm at 30 °C | 60.75 dyn/cm at 90 °C |
| 70.38 dyn/cm at 35 °C | 58.85 dyn/cm at 100 °C |
| Temperature, °C | Conductivity, μS/m |
|---|---|
| 0.01 | 1.15 |
| 25 | 5.50 |
| 100 | 76.5 |
| 200 | 299 |
| 300 | 241 |
Water/steam equilibrium properties
Vapor pressure formula for steam in equilibrium with liquid water:[14]
where P is equilibrium vapor pressure in kPa, and T is temperature in kelvins.
For T = 273 K to 333 K: A = 7.2326; B = 1750.286; C = 38.1.
For T = 333 K to 423 K: A = 7.0917; B = 1668.21; C = 45.1.
| Steam table[15] | |||||
|---|---|---|---|---|---|
| Temperature (°C) | Pressure (kPa) | H of liquid (J/g) | ΔvapH (J/g) | Wvap (J/g) | ρ of vapor (kg/m3) |
| 0 | 0.612 | 0.00 | 2496.5 | 126.0 | 0.004845 |
| 10 | 1.227 | 42.0 | 2473.5 | 130.5 | 0.009398 |
| 20 | 2.336 | 83.8 | 2450.9 | 135.1 | 0.01728 |
| 30 | 4.242 | 125.6 | 2427.9 | 139.7 | 0.03036 |
| 40 | 7.370 | 167.2 | 2404.9 | 144.2 | 0.05107 |
| 50 | 12.33 | 209.0 | 2381.4 | 148.7 | 0.08285 |
| 60 | 19.90 | 250.8 | 2357.6 | 153.0 | 0.1300 |
| 70 | 31.15 | 292.7 | 2332.9 | 157.3 | 0.1979 |
| 80 | 46.12 | 334.6 | 2307.7 | 161.5 | 0.2931 |
| 90 | 70.10 | 376.6 | 2282.6 | 165.5 | 0.4232 |
| 100 | 101.32 | 419.0 | 2256.3 | 169.4 | 0.5974 |
| 110 | 143.27 | 460.8 | 2229.5 | 173.1 | 0.8264 |
| 120 | 198.50 | 503.2 | 2201.4 | 176.7 | 1.121 |
| 130 | 270.13 | 545.8 | 2172.5 | 180.2 | 1.497 |
| 140 | 361.4 | 588.5 | 2142.8 | 183.2 | 1.967 |
| 150 | 476.0 | 631.5 | 2111.8 | 186.1 | 2.548 |
| 160 | 618.1 | 674.7 | 2080.0 | 188.7 | 3.263 |
| 170 | 792.0 | 718.5 | 2047.0 | 190.6 | 4.023 |
| 180 | 1002.7 | 762.5 | 2012.2 | 192.8 | 5.165 |
| 190 | 1254.9 | 807.0 | 1975.8 | 194.5 | 6.402 |
| 200 | 1554.3 | 851.9 | 1937.3 | 195.6 | 7.868 |
| 210 | 1907.9 | 897.5 | 1897.5 | 196.3 | 9.606 |
| 221.1 | 2369.8 | 948.5 | 1850.2 | 196.6 | 11.88 |
| 229.4 | 2769.6 | 987.9 | 1812.5 | 196.2 | 13.87 |
| 240.6 | 3381.1 | 1040.6 | 1759.4 | 195.1 | 16.96 |
| 248.9 | 3904.1 | 1080.3 | 1715.8 | 193.7 | 19.66 |
| 260.0 | 4695.9 | 1134.8 | 1653.9 | 190.8 | 23.84 |
| 271.1 | 5603.4 | 1195.9 | 1586.5 | 186.9 | 28.83 |
| 279.4 | 6366.5 | 1240.7 | 1532.5 | 183.3 | 33.18 |
| 290.6 | 7506.2 | 1302.3 | 1456.3 | 177.4 | 39.95 |
| 298.9 | 8463.9 | 1350.0 | 1394.8 | 172.2 | 45.93 |
| 310.0 | 9878.0 | 1415.7 | 1307.7 | 164.2 | 55.25 |
| 321.1 | 11461 | 1483.9 | 1212.7 | 154.5 | 66.58 |
| 329.4 | 12785 | 1537.9 | 1133.2 | 145.6 | 76.92 |
| 340.6 | 14727 | 1617.9 | 1007.6 | 130.9 | 94.25 |
| 348.9 | 16331 | 1687.0 | 892.0 | 117.0 | 111.5 |
| 360.0 | 18682 | 1797.0 | 694.0 | 91.0 | 145.3 |
| 371.1 | 21349 | 1968.3 | 365.0 | 47.0 | 214.5 |
| 374.4 | 22242 | 2151.2 | 0 | 0 | 306.8 |
| Temperature (°C) | Pressure (kPa) | H of liquid (J/g) | ΔvapH (J/g) | Wvap (J/g) | ρ of vapor (kg/m3) |
Data in the table above is given for water–steam equilibria at various temperatures over the entire temperature range at which liquid water can exist. Pressure of the equilibrium is given in the second column in kPa. The third column is the heat content of each gram of the liquid phase relative to water at 0 °C. The fourth column is the heat of vaporization of each gram of liquid that changes to vapor. The fifth column is the work PΔV done by each gram of liquid that changes to vapor. The sixth column is the density of the vapor.
Melting point of ice at various pressures
Data obtained from CRC Handbook of Chemistry and Physics 44th ed., p. 2390
| Pressure kPa | Temp. °C |
| 101.325 | 0.0 |
| 32950 | −2.5 |
| 60311 | −5.0 |
| 87279 | −7.5 |
| 113267 | −10.0 |
| 138274 | −12.5 |
| 159358 | −15.0 |
| 179952 | −17.5 |
| 200251 | −20.0 |
| 215746 | −22.1 |
Table of various forms of ice
| Properties of various forms of ice[16] | |||||
|---|---|---|---|---|---|
| Ice form | Density g/cm3 | Crystal structure | Triple points | TP temp °C | TP pressure MPa |
| Ih | 0.92 | hexagonal | Lq, Vap, Ih | 0.01 | 0.000612 |
| Lq, Ih, III | −22.0 | 207.5 | |||
| Ih, II, III | −34.7 | 212.9 | |||
| Ic | 0.92 | cubic | |||
| II | 1.17 | rhombohedral | Ih, II, III | −34.7 | 212.9 |
| II, III, V | −24.3 | 344.3 | |||
| II, V, VI | −55 (est) | 620 | |||
| III | 1.14 | tetragonal | Lq, Ih, III | −22.0 | 207.5 |
| Lq, III, V | −17 | 346.3 | |||
| Ih, II, III | −34.7 | 212.9 | |||
| II, III, V | −24.3 | 344.3 | |||
| IV | 1.27 | rhombohedral | |||
| V | 1.23 | monoclinic | Lq, III, V | −17 | 346.3 |
| Lq, V, VI | 0.16 | 625.9 | |||
| II, III, V | −24.3 | 344.3 | |||
| II, V, VI | −55 (est) | 620 | |||
| VI | 1.31 | tetragonal | Lq, V, VI | 0.16 | 625.9 |
| Lq, VI, VII | 81.6 | 2200 | |||
| II, V, VI | −55 (est) | 620 | |||
| VI, VII, VIII | ≈5 | 2100 | |||
| VII | 1.50 | cubic | Lq, VI, VII | 81.6 | 2200 |
| VI, VII, VIII | ≈5 | 2100 | |||
| VII, VIII, X | −173 | 62000 | |||
| VIII | 1.46 | tetragonal | VI, VII, VIII | ≈5 | 2100 |
| VII, VIII, X | −173 | 62000 | |||
| IX | 1.16 | tetragonal | |||
| X | 2.46 | cubic | VII, VIII, X | −173 | 62000 |
| XI‡ | 0.92 | orthorhombic | Vap, Ih, XI | −201.5 | 0 (expected) |
| XII | 1.29 | tetragonal | |||
| XIII | 1.23 | monoclinic | |||
| XIV | 1.29 | orthorhombic | |||
Phase diagram
Water with dissolved NaCl
| NaCl, wt% | Teq, °C | ρ, g/cm3 | n | η, mPa·s |
|---|---|---|---|---|
| 0 | 0 | 0.99984 | 1.333 | 1.002 |
| 0.5 | −0.3 | 1.0018 | 1.3339 | 1.011 |
| 1 | −0.59 | 1.0053 | 1.3347 | 1.02 |
| 2 | −1.19 | 1.0125 | 1.3365 | 1.036 |
| 3 | −1.79 | 1.0196 | 1.3383 | 1.052 |
| 4 | −2.41 | 1.0268 | 1.34 | 1.068 |
| 5 | −3.05 | 1.034 | 1.3418 | 1.085 |
| 6 | −3.7 | 1.0413 | 1.3435 | 1.104 |
| 7 | −4.38 | 1.0486 | 1.3453 | 1.124 |
| 8 | −5.08 | 1.0559 | 1.347 | 1.145 |
| 9 | −5.81 | 1.0633 | 1.3488 | 1.168 |
| 10 | −6.56 | 1.0707 | 1.3505 | 1.193 |
| 12 | −8.18 | 1.0857 | 1.3541 | 1.25 |
| 14 | −9.94 | 1.1008 | 1.3576 | 1.317 |
| 16 | −11.89 | 1.1162 | 1.3612 | 1.388 |
| 18 | −14.04 | 1.1319 | 1.3648 | 1.463 |
| 20 | −16.46 | 1.1478 | 1.3684 | 1.557 |
| 22 | −19.18 | 1.164 | 1.3721 | 1.676 |
| 23.3 | −21.1 | |||
| 23.7 | −17.3 | |||
| 24.9 | −11.1 | |||
| 26.1 | −2.7 | |||
| 26.28 | 0 | |||
| 26.32 | 10 | |||
| 26.41 | 20 | |||
| 26.45 | 25 | |||
| 26.52 | 30 | |||
| 26.67 | 40 | |||
| 26.84 | 50 | |||
| 27.03 | 60 | |||
| 27.25 | 70 | |||
| 27.5 | 80 | |||
| 27.78 | 90 | |||
| 28.05 | 100 |
Note: ρ is density, n is refractive index at 589 nm,{{clarify|date=May 2018}} and η is viscosity, all at 20 °C; Teq is the equilibrium temperature between two phases: ice/liquid solution for Teq < 0–0.1 °C and NaCl/liquid solution for Teq above 0.1 °C.
Self-ionization
{{Main article|Self-ionization of water}}| °C | −35 | 0 | 25 | 60 | 300 (~50 MPa) |
|---|---|---|---|---|---|
| pKw[18] | 17 | 14.9 | 14.0 | 13.0 | 12 |
==Spectral data==
| UV-Vis | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| λmax | ? nm | ||||||||||||||
| Extinction coefficient, ε | ? | ||||||||||||||
| IR | |||||||||||||||
Major absorption bands[19]
| |||||||||||||||
| NMR | |||||||||||||||
| Proton NMR | 4.79 ppm in D2O ; 1.56 ppm in CDCl3 ; 0.40 ppm in C6D6 ; 4.87 in CD3OD[20] | ||||||||||||||
| Carbon-13 NMR | N/A | ||||||||||||||
| Other NMR data | |||||||||||||||
| MS | |||||||||||||||
| Masses of main fragments | |||||||||||||||
Self-diffusion coefficients
| Experimental self-diffusion coefficients at various temperatures[21] | ||||||||||
| Temperature in {{formatnum:}}°C | -9}} m2/s | |||||||||
| 0 | 1.099 | |||||||||
| 1 | 1.138 | |||||||||
| 4 | 1.261 | |||||||||
| 5 | 1.303 | |||||||||
| 10 | 1.525 | |||||||||
| 15 | 1.765 | |||||||||
| 20 | 2.023 | |||||||||
| 25 | 2.299 | |||||||||
| 30 | 2.594 | |||||||||
| 35 | 2.907 | |||||||||
| 40 | 3.238 | |||||||||
| 45 | 3.588 | |||||||||
| 50 | 3.956 | |||||||||
| 56 | 4.423 | |||||||||
| 60 | 4.748 | |||||||||
| 70 | 5.615 | |||||||||
| 80 | 6.557 | |||||||||
| 90 | 7.574 | |||||||||
| 100 | 8.667 | |||||||||
Additional data translated from German "Wasser (Stoffdaten)" page
The data that follows was copied and translated from the German language Wikipedia version of this page (which has moved to here). It provides supplementary physical, thermodynamic, and vapor pressure data, some of which is redundant with data in the tables above, and some of which is additional.
Physical and thermodynamic tables
In the following tables, values are temperature-dependent and to a lesser degree pressure-dependent, and are arranged by state of aggregation (s = solid, lq = liquid, g = gas), which are clearly a function of temperature and pressure. All of the data were computed from data given in "Formulation of the Thermodynamic Properties of Ordinary Water Substance for Scientific and General Use" (1984). This applies to:
- T – temperature in degrees Celsius
- V – specific volume in cubic decimeters per kilogram (1 dm3 is equivalent to 1 liter)
- H – specific enthalpy in kilojoules per kilogram
- U – specific internal energy in kilojoules per kilogram
- S – specific entropy in kilojoules per kilogram-kelvin
- cp – specific heat capacity at constant pressure in kilojoules per kilogram-kelvin
- γ – Thermal expansion coefficient as 10−3 per kelvin
- λ – Heat conductivity in milliwatts per meter-kelvin
- η – Viscosity in micropascal-seconds (1 cP = 1000 µPa·s)
- σ – surface tension in millinewtons per meter (equivalent to dyn/cm)
Standard conditions
In the following table, material data are given for standard pressure of 0.1 MPa (equivalent to 1 bar). Up to 99.63 °C (the boiling point of water at 0.1 MPa), at this pressure water exists as a liquid. Above that, it exists as water vapor. Note that the boiling point of 100.0 °C is at a pressure of 0.101325 MPa (1 atm), which is the average atmospheric pressure.
| Water/steam data table at standard pressure (0.1 MPa) | ||||||||||
| T °C | V dm3/kg | H kJ/kg | U kJ/kg | S kJ/(kg·K) | cp kJ/(kg·K) | γ 10−3/K | λ mW / (m·K) | η µPa·s | σ ‡ mN/m | |
| 0 | lq | 1.0002 | 0.06 | −0.04 | −0.0001 | 4.228 | −0.080 | 561.0 | 1792 | 75.65 |
| 5 | 1.0000 | 21.1 | 21.0 | 0.076 | 4.200 | 0.011 | 570.6 | 1518 | 74.95 | |
| 10 | 1.0003 | 42.1 | 42.0 | 0.151 | 4.188 | 0.087 | 580.0 | 1306 | 74.22 | |
| 15 | 1.0009 | 63.0 | 62.9 | 0.224 | 4.184 | 0.152 | 589.4 | 1137 | 73.49 | |
| 20 | 1.0018 | 83.9 | 83.8 | 0.296 | 4.183 | 0.209 | 598.4 | 1001 | 72.74 | |
| 25 | 1.0029 | 104.8 | 104.7 | 0.367 | 4.183 | 0.259 | 607.2 | 890.4 | 71.98 | |
| 30 | 1.0044 | 125.8 | 125.7 | 0.437 | 4.183 | 0.305 | 615.5 | 797.7 | 71.20 | |
| 35 | 1.0060 | 146.7 | 146.6 | 0.505 | 4.183 | 0.347 | 623.3 | 719.6 | 70.41 | |
| 40 | 1.0079 | 167.6 | 167.5 | 0.572 | 4.182 | 0.386 | 630.6 | 653.3 | 69.60 | |
| 45 | 1.0099 | 188.5 | 188.4 | 0.638 | 4.182 | 0.423 | 637.3 | 596.3 | 68.78 | |
| 50 | 1.0121 | 209.4 | 209.3 | 0.704 | 4.181 | 0.457 | 643.6 | 547.1 | 67.95 | |
| 60 | 1.0171 | 251.2 | 251.1 | 0.831 | 4.183 | 0.522 | 654.4 | 466.6 | 66.24 | |
| 70 | 1.0227 | 293.1 | 293.0 | 0.955 | 4.187 | 0.583 | 663.1 | 404.1 | 64.49 | |
| 80 | 1.0290 | 335.0 | 334.9 | 1.075 | 4.194 | 0.640 | 670.0 | 354.5 | 62.68 | |
| 90 | 1.0359 | 377.0 | 376.9 | 1.193 | 4.204 | 0.696 | 675.3 | 314.6 | 60.82 | |
| 99.63 | lq | 1.0431 | 417.5 | 417.4 | 1.303 | 4.217 | 0.748 | 679.0 | 283.0 | 58.99 |
| g | 1694.3 | 2675 | 2505 | 7.359 | 2.043 | 2.885 | 25.05 | 12.26 | – | |
| 100 | g | 1696.1 | 2675 | 2506 | 7.361 | 2.042 | 2.881 | 25.08 | 12.27 | 58.92 |
| 200 | 2172.3 | 2874 | 2657 | 7.833 | 1.975 | 2.100 | 33.28 | 16.18 | 37.68 | |
| 300 | 2638.8 | 3073 | 2810 | 8.215 | 2.013 | 1.761 | 43.42 | 20.29 | 14.37 | |
| 500 | 3565.5 | 3488 | 3131 | 8.834 | 2.135 | 1.297 | 66.970 | 28.57 | – | |
| 750 | 4721.0 | 4043 | 3571 | 9.455 | 2.308 | 0.978 | 100.30 | 38.48 | – | |
| 1000 | 5875.5 | 4642 | 4054 | 9.978 | 2.478 | 0.786 | 136.3 | 47.66 | – | |
| ‡ The values for surface tension for the liquid section of the table are for a liquid/air interface. Values for the gas section of the table are for a liquid/saturated steam interface. | ||||||||||
Triple point
In the following table, material data are given with a pressure of 611.7 Pa (equivalent to 0.006117 bar). Up to a temperature of 0.01 °C, the triple point of water, water normally exists as ice, except for supercooled water, for which one data point is tabulated here. At the triple point, ice can exist together with both liquid water and vapor. At higher temperatures, the data are for water vapor only.
| Water/steam data table at triple point pressure (0.0006117 MPa) | |||||||||
| T °C | V dm3/kg | H kJ/kg | U kJ/kg | S kJ/(kg·K) | cp kJ/(kg·K) | γ 10−3/K | λ mW / (m·K) | η µPa·s | |
| 0 | lq | 1.0002 | −0.04 | −0.04 | −0.0002 | 4.339 | −0.081 | 561.0 | 1792 |
| 0.01 | s | 1.0908 | −333.4 | −333.4 | −1.221 | 1.93 | 0.1 | 2180 | – |
| lq | 1.0002 | 0.0 | 0 | 0 | 4.229 | −0.080 | 561.0 | 1791 | |
| g | 205986 | 2500 | 2374 | 9.154 | 1.868 | 3.672 | 17.07 | 9.22 | |
| 5 | g | 209913 | 2509 | 2381 | 9.188 | 1.867 | 3.605 | 17.33 | 9.34 |
| 10 | 213695 | 2519 | 2388 | 9.222 | 1.867 | 3.540 | 17.60 | 9.46 | |
| 15 | 217477 | 2528 | 2395 | 9.254 | 1.868 | 3.478 | 17.88 | 9.59 | |
| 20 | 221258 | 2537 | 2402 | 9.286 | 1.868 | 3.417 | 18.17 | 9.73 | |
| 25 | 225039 | 2547 | 2409 | 9.318 | 1.869 | 3.359 | 18.47 | 9.87 | |
| 30 | 228819 | 2556 | 2416 | 9.349 | 1.869 | 3.304 | 18.78 | 10.02 | |
| 35 | 232598 | 2565 | 2423 | 9.380 | 1.870 | 3.249 | 19.10 | 10.17 | |
| 40 | 236377 | 2575 | 2430 | 9.410 | 1.871 | 3.197 | 19.43 | 10.32 | |
| 45 | 240155 | 2584 | 2437 | 9.439 | 1.872 | 3.147 | 19.77 | 10.47 | |
| 50 | 243933 | 2593 | 2444 | 9.469 | 1.874 | 3.098 | 20.11 | 10.63 | |
| 60 | 251489 | 2612 | 2459 | 9.526 | 1.876 | 3.004 | 20.82 | 10.96 | |
| 70 | 259043 | 2631 | 2473 | 9.581 | 1.880 | 2.916 | 21.56 | 11.29 | |
| 80 | 266597 | 2650 | 2487 | 9.635 | 1.883 | 2.833 | 22.31 | 11.64 | |
| 90 | 274150 | 2669 | 2501 | 9.688 | 1.887 | 2.755 | 23.10 | 11.99 | |
| 100 | 281703 | 2688 | 2515 | 9.739 | 1.891 | 2.681 | 23.90 | 12.53 | |
| 200 | 357216 | 2879 | 2661 | 10.194 | 1.940 | 2.114 | 32.89 | 16.21 | |
| 300 | 432721 | 3076 | 2811 | 10.571 | 2.000 | 1.745 | 43.26 | 20.30 | |
| 500 | 583725 | 3489 | 3132 | 11.188 | 2.131 | 1.293 | 66.90 | 28.57 | |
| 750 | 772477 | 4043 | 3571 | 11.808 | 2.307 | 0.977 | 100.20 | 38.47 | |
| 1000 | 961227 | 4642 | 4054 | 12.331 | 2.478 | 0.785 | 136.30 | 47.66 | |
Saturated vapor pressure
The following table is based on different, complementary sources and approximation formulas, whose values are of various quality and accuracy. The values in the temperature range of −100 °C to 100 °C were inferred from D. Sunday (1982) and are quite uniform and exact. The values in the temperature range of the boiling point of water up to the critical point (100 °C to 374 °C) are drawn from different sources and are substantially less accurate; hence they should be used only as approximate values.[22][23][24][25]
To use the values correctly, consider the following points:
- The values apply only to smooth interfaces and in the absence other gases or gas mixtures such as air. Hence they apply only to pure phases and need a correction factor for systems in which air is present.
- The values were not computed according formulas widely used in the US, but using somewhat more exact formulas (see below), which can also be used to compute further values in the appropriate temperature ranges.
- The saturated vapor pressure over water in the temperature range of −100 °C to −50 °C is only extrapolated [Translator's note: Supercooled liquid water is not known to exist below −42 °C].
- The values have various units (Pa, hPa or bar), which must be considered when reading them.
Formulas
The table values for −100 °C to 100 °C were computed by the following formulas, where T is in kelvins and vapor pressures, Pw and Pi, are in pascals.
Over liquid waterloge(Pw) = −6094.4642 T−1 + 21.1249952 − 2.724552×10−2 T + 1.6853396×10−5 T2 + 2.4575506 loge(T)
For temperature range: 173.15 K to 373.15 K or equivalently −100 °C to 100 °C
Over iceloge(Pi) = −5504.4088 T−1 − 3.5704628 − 1.7337458×10−2 T + 6.5204209×10−6 T2 + 6.1295027 loge(T)
For temperature range: 173.15 K to 273.15 K or equivalently −100 °C to 0 °C
At triple pointAn important basic value, which is not registered in the table, is the saturated vapor pressure at the triple point of water. The internationally accepted value according to measurements of Guildner, Johnson and Jones (1976) amounts to:
Pw(ttp = 0.01 °C) = 611.657 Pa ± 0.010 Pa at (1 − α) = 99%
| Values of saturated vapor pressure of water | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temp. T in °C | Pi(T) over ice in Pa | Pw(T) over water in Pa | Temp. T in °C | Pw(T) over water in hPa | Temp. T in °C | P(T) in bar | Temp. T in °C | P(T) in bar | Temp. T in °C | P(T) in bar | ||||
| −100 | 0.0013957 | 0.0036309 | 0 | 6.11213 | 100 | 1.01 | 200 | 15.55 | 300 | 85.88 | ||||
| −99 | 0.0017094 | 0.0044121 | 1 | 6.57069 | 101 | 1.05 | 201 | 15.88 | 301 | 87.09 | ||||
| −98 | 0.0020889 | 0.0053487 | 2 | 7.05949 | 102 | 1.09 | 202 | 16.21 | 302 | 88.32 | ||||
| −97 | 0.0025470 | 0.0064692 | 3 | 7.58023 | 103 | 1.13 | 203 | 16.55 | 303 | 89.57 | ||||
| −96 | 0.0030987 | 0.0078067 | 4 | 8.13467 | 104 | 1.17 | 204 | 16.89 | 304 | 90.82 | ||||
| −95 | 0.0037617 | 0.0093996 | 5 | 8.72469 | 105 | 1.21 | 205 | 17.24 | 305 | 92.09 | ||||
| −94 | 0.0045569 | 0.011293 | 6 | 9.35222 | 106 | 1.25 | 206 | 17.60 | 306 | 93.38 | ||||
| −93 | 0.0055087 | 0.013538 | 7 | 10.0193 | 107 | 1.30 | 207 | 17.96 | 307 | 94.67 | ||||
| −92 | 0.0066455 | 0.016195 | 8 | 10.7280 | 108 | 1.34 | 208 | 18.32 | 308 | 95.98 | ||||
| −91 | 0.0080008 | 0.019333 | 9 | 11.4806 | 109 | 1.39 | 209 | 18.70 | 309 | 97.31 | ||||
| −90 | 0.0096132 | 0.023031 | 10 | 12.2794 | 110 | 1.43 | 210 | 19.07 | 310 | 98.65 | ||||
| −89 | 0.011528 | 0.027381 | 11 | 13.1267 | 111 | 1.48 | 211 | 19.46 | 311 | 100.00 | ||||
| −88 | 0.013797 | 0.032489 | 12 | 14.0251 | 112 | 1.53 | 212 | 19.85 | 312 | 101.37 | ||||
| −87 | 0.016482 | 0.038474 | 13 | 14.9772 | 113 | 1.58 | 213 | 20.25 | 313 | 102.75 | ||||
| −86 | 0.019653 | 0.045473 | 14 | 15.9856 | 114 | 1.64 | 214 | 20.65 | 314 | 104.15 | ||||
| −85 | 0.02339 | 0.053645 | 15 | 17.0532 | 115 | 1.69 | 215 | 21.06 | 315 | 105.56 | ||||
| −84 | 0.027788 | 0.063166 | 16 | 18.1829 | 116 | 1.75 | 216 | 21.47 | 316 | 106.98 | ||||
| −83 | 0.032954 | 0.074241 | 17 | 19.3778 | 117 | 1.81 | 217 | 21.89 | 317 | 108.43 | ||||
| −82 | 0.039011 | 0.087101 | 18 | 20.6409 | 118 | 1.86 | 218 | 22.32 | 318 | 109.88 | ||||
| −81 | 0.046102 | 0.10201 | 19 | 21.9757 | 119 | 1.93 | 219 | 22.75 | 319 | 111.35 | ||||
| −80 | 0.054388 | 0.11925 | 20 | 23.3854 | 120 | 1.99 | 220 | 23.19 | 320 | 112.84 | ||||
| −79 | 0.064057 | 0.13918 | 21 | 24.8737 | 121 | 2.05 | 221 | 23.64 | 321 | 114.34 | ||||
| −78 | 0.075320 | 0.16215 | 22 | 26.4442 | 122 | 2.12 | 222 | 24.09 | 322 | 115.86 | ||||
| −77 | 0.088419 | 0.18860 | 23 | 28.1006 | 123 | 2.18 | 223 | 24.55 | 323 | 117.39 | ||||
| −76 | 0.10363 | 0.21901 | 24 | 29.8470 | 124 | 2.25 | 224 | 25.02 | 324 | 118.94 | ||||
| −75 | 0.12127 | 0.25391 | 25 | 31.6874 | 125 | 2.32 | 225 | 25.49 | 325 | 120.51 | ||||
| −74 | 0.14168 | 0.29390 | 26 | 33.6260 | 126 | 2.40 | 226 | 25.98 | 326 | 122.09 | ||||
| −73 | 0.16528 | 0.33966 | 27 | 35.6671 | 127 | 2.47 | 227 | 26.46 | 327 | 123.68 | ||||
| −72 | 0.19252 | 0.39193 | 28 | 37.8154 | 128 | 2.55 | 228 | 26.96 | 328 | 125.30 | ||||
| −71 | 0.22391 | 0.45156 | 29 | 40.0754 | 129 | 2.62 | 229 | 27.46 | 329 | 126.93 | ||||
| −70 | 0.26004 | 0.51948 | 30 | 42.4520 | 130 | 2.70 | 230 | 27.97 | 330 | 128.58 | ||||
| −69 | 0.30156 | 0.59672 | 31 | 44.9502 | 131 | 2.78 | 231 | 28.48 | 331 | 130.24 | ||||
| −68 | 0.34921 | 0.68446 | 32 | 47.5752 | 132 | 2.87 | 232 | 29.01 | 332 | 131.92 | ||||
| −67 | 0.40383 | 0.78397 | 33 | 50.3322 | 133 | 2.95 | 233 | 29.54 | 333 | 133.62 | ||||
| −66 | 0.46633 | 0.89668 | 34 | 53.2267 | 134 | 3.04 | 234 | 30.08 | 334 | 135.33 | ||||
| −65 | 0.53778 | 1.0242 | 35 | 56.2645 | 135 | 3.13 | 235 | 30.62 | 335 | 137.07 | ||||
| −64 | 0.61933 | 1.1682 | 36 | 59.4513 | 136 | 3.22 | 236 | 31.18 | 336 | 138.82 | ||||
| −63 | 0.71231 | 1.3306 | 37 | 62.7933 | 137 | 3.32 | 237 | 31.74 | 337 | 140.59 | ||||
| −62 | 0.81817 | 1.5136 | 38 | 66.2956 | 138 | 3.42 | 238 | 32.31 | 338 | 142.37 | ||||
| −61 | 0.93854 | 1.7195 | 39 | 69.9675 | 139 | 3.51 | 239 | 32.88 | 339 | 144.18 | ||||
| −60 | 1.0753 | 1.9509 | 40 | 73.8127 | 140 | 3.62 | 240 | 33.47 | 340 | 146.00 | ||||
| −59 | 1.2303 | 2.2106 | 41 | 77.8319 | 141 | 3.72 | 241 | 34.06 | 341 | 147.84 | ||||
| −58 | 1.4060 | 2.5018 | 42 | 82.0536 | 142 | 3.82 | 242 | 34.66 | 342 | 149.71 | ||||
| −57 | 1.6049 | 2.8277 | 43 | 86.4633 | 143 | 3.93 | 243 | 35.27 | 343 | 151.58 | ||||
| −56 | 1.8296 | 3.1922 | 44 | 91.0757 | 144 | 4.04 | 244 | 35.88 | 344 | 153.48 | ||||
| −55 | 2.0833 | 3.5993 | 45 | 95.8984 | 145 | 4.16 | 245 | 36.51 | 345 | 155.40 | ||||
| −54 | 2.3694 | 4.0535 | 46 | 100.939 | 146 | 4.27 | 246 | 37.14 | 346 | 157.34 | ||||
| −53 | 2.6917 | 4.5597 | 47 | 106.206 | 147 | 4.39 | 247 | 37.78 | 347 | 159.30 | ||||
| −52 | 3.0542 | 5.1231 | 48 | 111.708 | 148 | 4.51 | 248 | 38.43 | 348 | 161.28 | ||||
| −51 | 3.4618 | 5.7496 | 49 | 117.452 | 149 | 4.64 | 249 | 39.09 | 349 | 163.27 | ||||
| −50 | 3.9193 | 6.4454 | 50 | 123.4478 | 150 | 4.76 | 250 | 39.76 | 350 | 165.29 | ||||
| −49 | 4.4324 | 7.2174 | 51 | 129.7042 | 151 | 4.89 | 251 | 40.44 | 351 | 167.33 | ||||
| −48 | 5.0073 | 8.0729 | 52 | 136.2304 | 152 | 5.02 | 252 | 41.12 | 352 | 169.39 | ||||
| −47 | 5.6506 | 9.0201 | 53 | 143.0357 | 153 | 5.16 | 253 | 41.81 | 353 | 171.47 | ||||
| −46 | 6.3699 | 10.068 | 54 | 150.1298 | 154 | 5.29 | 254 | 42.52 | 354 | 173.58 | ||||
| −45 | 7.1732 | 11.225 | 55 | 157.5226 | 155 | 5.43 | 255 | 43.23 | 355 | 175.70 | ||||
| −44 | 8.0695 | 12.503 | 56 | 165.2243 | 156 | 5.58 | 256 | 43.95 | 356 | 177.85 | ||||
| −43 | 9.0685 | 13.911 | 57 | 173.2451 | 157 | 5.72 | 257 | 44.68 | 357 | 180.02 | ||||
| −42 | 10.181 | 15.463 | 58 | 181.5959 | 158 | 5.87 | 258 | 45.42 | 358 | 182.21 | ||||
| −41 | 11.419 | 17.170 | 59 | 190.2874 | 159 | 6.03 | 259 | 46.16 | 359 | 184.43 | ||||
| −40 | 12.794 | 19.048 | 60 | 199.3309 | 160 | 6.18 | 260 | 46.92 | 360 | 186.66 | ||||
| −39 | 14.321 | 21.110 | 61 | 208.7378 | 161 | 6.34 | 261 | 47.69 | 361 | 188.93 | ||||
| −38 | 16.016 | 23.372 | 62 | 218.5198 | 162 | 6.50 | 262 | 48.46 | 362 | 191.21 | ||||
| −37 | 17.893 | 25.853 | 63 | 228.6888 | 163 | 6.67 | 263 | 49.25 | 363 | 193.52 | ||||
| −36 | 19.973 | 28.570 | 64 | 239.2572 | 164 | 6.84 | 264 | 50.05 | 364 | 195.86 | ||||
| −35 | 22.273 | 31.544 | 65 | 250.2373 | 165 | 7.01 | 265 | 50.85 | 365 | 198.22 | ||||
| −34 | 24.816 | 34.795 | 66 | 261.6421 | 166 | 7.18 | 266 | 51.67 | 366 | 200.61 | ||||
| −33 | 27.624 | 38.347 | 67 | 273.4845 | 167 | 7.36 | 267 | 52.49 | 367 | 203.02 | ||||
| −32 | 30.723 | 42.225 | 68 | 285.7781 | 168 | 7.55 | 268 | 53.33 | 368 | 205.47 | ||||
| −31 | 34.140 | 46.453 | 69 | 298.5363 | 169 | 7.73 | 269 | 54.17 | 369 | 207.93 | ||||
| −30 | 37.903 | 51.060 | 70 | 311.7731 | 170 | 7.92 | 270 | 55.03 | 370 | 210.43 | ||||
| −29 | 42.046 | 56.077 | 71 | 325.5029 | 171 | 8.11 | 271 | 55.89 | 371 | 212.96 | ||||
| −28 | 46.601 | 61.534 | 72 | 339.7401 | 172 | 8.31 | 272 | 56.77 | 372 | 215.53 | ||||
| −27 | 51.607 | 67.466 | 73 | 354.4995 | 173 | 8.51 | 273 | 57.66 | 373 | 218.13 | ||||
| −26 | 57.104 | 73.909 | 74 | 369.7963 | 174 | 8.72 | 274 | 58.56 | 374 | 220.64 | ||||
| −25 | 63.134 | 80.902 | 75 | 385.6459 | 175 | 8.92 | 275 | 59.46 | 374.15 | 221.20 | ||||
| −24 | 69.745 | 88.485 | 76 | 402.0641 | 176 | 9.14 | 276 | 60.38 | ||||||
| −23 | 76.987 | 96.701 | 77 | 419.0669 | 177 | 9.35 | 277 | 61.31 | ||||||
| −22 | 84.914 | 105.60 | 78 | 436.6708 | 178 | 9.57 | 278 | 62.25 | ||||||
| −21 | 93.584 | 115.22 | 79 | 454.8923 | 179 | 9.80 | 279 | 63.20 | ||||||
| −20 | 103.06 | 125.63 | 80 | 473.7485 | 180 | 10.03 | 280 | 64.17 | ||||||
| −19 | 113.41 | 136.88 | 81 | 493.2567 | 181 | 10.26 | 281 | 65.14 | ||||||
| −18 | 124.70 | 149.01 | 82 | 513.4345 | 182 | 10.50 | 282 | 66.12 | ||||||
| −17 | 137.02 | 162.11 | 83 | 534.3000 | 183 | 10.74 | 283 | 67.12 | ||||||
| −16 | 150.44 | 176.23 | 84 | 555.8714 | 184 | 10.98 | 284 | 68.13 | ||||||
| −15 | 165.06 | 191.44 | 85 | 578.1673 | 185 | 11.23 | 285 | 69.15 | ||||||
| −14 | 180.97 | 207.81 | 86 | 601.2068 | 186 | 11.49 | 286 | 70.18 | ||||||
| −13 | 198.27 | 225.43 | 87 | 625.0090 | 187 | 11.75 | 287 | 71.22 | ||||||
| −12 | 217.07 | 244.37 | 88 | 649.5936 | 188 | 12.01 | 288 | 72.27 | ||||||
| −11 | 237.49 | 264.72 | 89 | 674.9806 | 189 | 12.28 | 289 | 73.34 | ||||||
| −10 | 259.66 | 286.57 | 90 | 701.1904 | 190 | 12.55 | 290 | 74.42 | ||||||
| −9 | 283.69 | 310.02 | 91 | 728.2434 | 191 | 12.83 | 291 | 75.51 | ||||||
| −8 | 309.75 | 335.16 | 92 | 756.1608 | 192 | 13.11 | 292 | 76.61 | ||||||
| −7 | 337.97 | 362.10 | 93 | 784.9639 | 193 | 13.40 | 293 | 77.72 | ||||||
| −6 | 368.52 | 390.95 | 94 | 814.6743 | 194 | 13.69 | 294 | 78.85 | ||||||
| −5 | 401.58 | 421.84 | 95 | 845.3141 | 195 | 13.99 | 295 | 79.99 | ||||||
| −4 | 437.31 | 454.88 | 96 | 876.9057 | 196 | 14.29 | 296 | 81.14 | ||||||
| −3 | 475.92 | 490.19 | 97 | 909.4718 | 197 | 14.60 | 297 | 82.31 | ||||||
| −2 | 517.62 | 527.93 | 98 | 943.0355 | 198 | 14.91 | 298 | 83.48 | ||||||
| −1 | 562.62 | 568.22 | 99 | 977.6203 | 199 | 15.22 | 299 | 84.67 | ||||||
| 0 | 611.153 | 611.213 | 100 | 1013.25 | 200 | 15.55 | 300 | 85.88 | ||||||
| Temp. T in °C | Pi(T) over ice in Pa | Pw(T) over water in Pa | Temp. T in °C | Pw(T) over water in hPa | Temp. T in °C | P(T) in bar | Temp. T in °C | P(T) in bar | Temp. T in °C | P(T) in bar | ||||
Magnetic susceptibility
Accepted standardized value of the magnetic susceptibility of water at 20 °C (room temperature) is −12.97 cm3/mol.[26]
Accepted standardized value of the magnetic susceptibility of water at 20 °C (room temperature) is −0.702 cm3/g.[26]
| Isotopolog, state | Temperature in K | Magnetic susceptibiliy in cm3/mol |
|---|---|---|
| H2O(g) | >373 | −13.1 |
| H2O(l) | 373 | −13.09 |
| H2O(l) | 293 | −12.97 |
| H2O(l) | 273 | −12.93 |
| H2O(s) | 273 | −12.65 |
| H2O(s) | 223 | −12.31 |
| DHO(l) | 302 | −12.97 |
| D2O(l) | 293 | −12.76 |
| D2O(l) | 276.8 | −12.66 |
| D2O(s) | 276.8 | −12.54 |
| D2O(s) | 213 | −12.41 |
References
{{Clear}}2. ^{{cite journal|doi=10.1063/1.2387163|title=A direct measurement of the dissociation energy of water|year=2006|last1=Maksyutenko|first1=Pavlo|last2=Rizzo|first2=Thomas R.|last3=Boyarkin|first3=Oleg V.|journal=The Journal of Chemical Physics|volume=125|pages=181101|pmid=17115729|issue=18|bibcode=2006JChPh.125r1101M}}
3. ^{{cite journal|doi=10.1016/0022-2852(74)90261-6|title=Molecular force field and structure of water: Recent microwave results|year=1974|last1=Cook|first1=R|last2=Delucia|first2=F|last3=Helminger|first3=P|journal=Journal of Molecular Spectroscopy|volume=53|pages=62|bibcode=1974JMoSp..53...62C}}
4. ^{{cite journal|year=1979|last1=Hoy|first1=AR|last2=Bunker|first2=PR|journal=Journal of Molecular Spectroscopy|volume=74|pages=1–8|doi=10.1016/0022-2852(79)90019-5|title=A precise solution of the rotation bending Schrödinger equation for a triatomic molecule with application to the water molecule|bibcode=1979JMoSp..74....1H}}
5. ^{{Cite web|url=http://cccbdb.nist.gov/expangle2.asp?descript=aHOH&all=0|title=List of experimental bond angles of type aHOH|website=Computational Chemistry Comparison and Benchmark DataBase|access-date=}}
6. ^Griffiths, D. J. "Introduction to Electrodynamics," 3rd Ed. page 275. Prentice Hall, 1999 {{ISBN|0-13-859851-7}}
7. ^{{cite web|url=http://www.engineeringtoolbox.com/sound-speed-water-d_598.html|title=Water and the Speed of Sound|publisher=www.engineeringtoolbox.com|accessdate=2008-04-29}}
8. ^Lange, p. 1199. Due to the old definition of liter used at the time, the data from the Handbook was converted from old g/ml to g/cm3, by multiplying by 0.999973.
9. ^{{Cite book|title=CRC Handbook of Chemistry and Physics|last=Lide|first=David R.|date=1989-06-30|publisher=CRC Press|isbn=978-0-849-30470-5|edition=70|location=Boca Raton, Florida|pages=}}{{page needed|date=June 2016}}
10. ^Franks, [https://books.google.at/books?id=5f_xBwAAQBAJ&pg=PA376&lpg=PA376&dq=999.972+3.984&source=bl&ots=cmB39QADE0&sig=PG_2dNaNirijB2QWF4XhRMFkk54&hl=en&sa=X&redir_esc=y#v=onepage&q=999.972%203.984&f=false Water a comprehensive treatise Volume 1 The Physics and Physical Chemistry of Water].
11. ^David R. Lide [https://books.google.com/books?id=WDll8hA006AC&pg=SA6-15 CRC Handbook of Chemistry and Physics] CRC Press, 2004, p. 6-201 {{ISBN|0-8493-0485-7}}.
12. ^Lange, p. 1663.
13. ^Revised Release on Viscosity and Thermal Conductivity of Heavy Water Substance, The International Association for the Properties of Water and Steam Lucerne, Switzerland, August 2007.
14. ^Lange, p. 1436.
15. ^Lange, p. 1476.
16. ^{{cite web|url=http://www.lsbu.ac.uk/water/phase.html|title=Water Phase Diagram|publisher=London South Bank University|author=Martin Chaplin|accessdate=2008-01-21}}
17. ^{{RubberBible86th|pages=8–71, 8–116}}
18. ^{{cite web|url=http://www.lsbu.ac.uk/water/ionis.html|title=Ionization of water|accessdate=2008-04-09|author=Martin Chaplin|publisher=London South Bank University}}
19. ^{{cite web|url=http://www.lsbu.ac.uk/water/vibrat.html|title=Water Absorption Spectrum|author=Martin Chaplin|publisher=London South Bank University|accessdate=2008-04-10}}
20. ^Fulmer et al. Organometallics, Vol. 29, No. 9, 2010
21. ^{{Cite journal|last=Holz|first=Manfred|last2=Heil|first2=Stefan R.|last3=Sacco|first3=Antonio|date=2000-01-01|title=Temperature-dependent self-diffusion coefficients of water and six selected molecular liquids for calibration in accurate {{chem|1|H}} NMR PFG measurements|url=http://xlink.rsc.org/?DOI=b005319h|journal=Physical Chemistry Chemical Physics|language=en|volume=2|issue=20|pages=4740–4742|bibcode=2000PCCP....2.4740H|doi=10.1039/b005319h|issn=1463-9084}}
22. ^{{cite journal|doi=10.1126/science.191.4233.1261|title=Vapor Pressure of Water at Its Triple Point: Highly Accurate Value|year=1976|last1=Guildner|first1=L. A.|last2=Johnson|first2=D. P.|last3=Jones|first3=F. E.|journal=Science|volume=191|pages=1261|pmid=17737716|issue=4233|bibcode=1976Sci...191.1261G}}
23. ^Klaus Scheffler (1981): Wasserdampftafeln: thermodynam. Eigenschaften von Wasser u. Wasserdampf bis 800°C u. 800 bar (Water Vapor Tables: Thermodynamic Characteristics of Water and Water Vapor to 800°C and 800 bar), Berlin [u.a.] {{ISBN|3-540-10930-7}}
24. ^D. Sonntag und D. Heinze (1982): Sättigungsdampfdruck- und Sättigungsdampfdichtetafeln für Wasser und Eis. (Saturated Vapor Pressure and Saturated Vapor Density Tables for Water and Ice)(1. Aufl.), VEB Deutscher Verlag für Grundstoffindustrie
25. ^Ulrich Grigull, Johannes Staub, Peter Schiebener (1990): Steam Tables in SI-Units – Wasserdampftafeln. Springer-Verlagdima gmbh
26. ^1 2 {{Cite book|title=CRC, Handbook of Chemistry and Physics 64th edition|last=Weast|first=Robert|publisher=CRC publishing|year=1983–1984|isbn=0-8493-0464-4|location=Boca Raton, Florida|pages=E-119|quote=|via=}}
Bibliography
- Lange's Handbook of Chemistry, 10th ed. {{ISBN|0-07-016384-7}} (for 15th edition)
- {{nist}}
External links
- Microwave Spectrum (by NIST)
- Compilation of properties, with citations by Martin Chaplin, London South Bank University.
- Embassy of Canada in France
- Embassy of Canada in Kabul
- Embassy of Canada in Mexico City
- Embassy of Canada in Moscow
- Embassy of Canada in Oslo
- Embassy of Canada in Paris
- Embassy of Canada in Tokyo
- Embassy of Canada in Washington
- Embassy of Canada in Washington, D.C.
- Embassy of Canada in Washington, DC
- Embassy of Canada, Kabul
- Embassy of Canada, Mexico City
- Embassy of Canada, Paris
- Embassy of Canada to Japan in Tokyo
- Embassy of Canada, Tokyo
- Embassy of Canada to Poland
- Embassy of Canada to Romania
- Embassy of Cape Verde, Washington, D.C.
- Embassy of Chile in Ottawa
- Embassy of China in Moscow
- Embassy of China in Ottawa
- Embassy of Colombia in Washington DC
- Embassy of Colombia, Washington, D.C.
- Embassy of Croatia in Moscow
- Embassy of Croatia in Ottawa