- See also
- References
}}
The Wiswesser rule gives a simple method to determine the energetic sequence of the atomic subshells 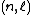 , where n is the principal quantum number and ℓ is the azimuthal quantum number. The energetic sequence of the subshells characterized by the quantum numbers
, where n is the principal quantum number and ℓ is the azimuthal quantum number. The energetic sequence of the subshells characterized by the quantum numbers 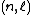 is the sequence that leads to a monotonically increasing row of function values for the Wiswesser function. The rule is named after William Wiswesser.
is the sequence that leads to a monotonically increasing row of function values for the Wiswesser function. The rule is named after William Wiswesser.
For example: if  and
and  , this corresponds to a 2p-orbital.
, this corresponds to a 2p-orbital.
If the results of this function for each shell and subshell are ordered, the resulting sequence is: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p...
To illustrate this, the corresponding values of each of these shells can be found in the table below:
 |  |  |
|---|---|---|
| 2 | 1 | 2.5 |
| 2 | 2 | 3.33 |
| 3 | 1 | 3.5 |
| 3 | 2 | 4.33 |
| 4 | 1 | 4.5 |
| 3 | 3 | 5.25 |
| 4 | 2 | 5.33 |
| 5 | 1 | 5.5 |
| 4 | 3 | 6.25 |
| 5 | 2 | 6.33 |
| 6 | 1 | 6.5 |
| 4 | 4 | 7.2 |
| 5 | 3 | 7.25 |
| 6 | 2 | 7.33 |
| 7 | 1 | 7.5 |
| 5 | 4 | 8.2 |
| 6 | 3 | 8.25 |
| 7 | 2 | 8.33 |
We can now clearly see that the aufbau principle and Wiswesser's rule come to the same order of filling electron shells.
See also
- Atomic orbital energy
- Aufbau principle
- Electron configuration
References
- D.C. v. Parker
- DC vs. Heller
- D.C. vs. Heller
- DC VS MK
- D.C. War Memorial
- DC Water
- DC Water and Sewer Authority
- D C Water and Sewer Authority
- D. C. Water and Sewer Authority
- DCW (disambiguation)
- D.C. Weller
- DC Weller (Coronation Street)
- DC Who's Who
- DC/Wildstorm: DreamWar
- D C Wimberly
- D.C. Wimberly
- DC Wimberly
- DCX (disambiguation)
- DCX (gene)
- DCXR
- D-Cycloserine
- DCYF
- DCypha Productions
- Dcypha Productions
- D.C.Z.
