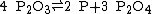- Preparation
- References
| Verifiedfields =
| Watchedfields =
| verifiedrevid =
| Name = Diphosphorus tetroxide
| ImageFileL1 =
| ImageSizeL1 = 150px
| ImageNameL1 =
| ImageFileR1 =
| ImageSizeR1 = 150px
| ImageNameR1 =
| ImageFile2 =
| ImageSize2 = 150
| OtherNames = Phosphorus tetroxide
Phosphorus(V) oxide
Phosphoric anhydride
|Section1={{Chembox Identifiers
| StdInChI =
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}}
| StdInChIKey =
| ChEBI_Ref =
| ChEBI =
| SMILES =
| CASNo_Ref = {{cascite|correct|CAS}}
| CASNo = 12137-38-1
| CASNo2_Ref = {{cascite|changed|??}}
| CASNo2 =
| CASNo2_Comment = (P4O10)
| PubChem =16131071
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}}
| ChemSpiderID =
| RTECS =
|Section2={{Chembox Properties
| Formula = P2O4
| MolarMass = 125.96 g·mol−1
| Appearance = Solid
| Density =
| Solubility =
| MeltingPt = >100 °C
| BoilingPtC =
| VaporPressure =2.54 g·cm−3
}}
|Section7={{Chembox Hazards
| ExternalSDS =
| EUClass = not listed
| NFPA-H =
| NFPA-R =
| NFPA-F =
| NFPA-S =
}}Diphosphorus tetroxide, or phosphorus tetroxide is an inorganic compound of phosphorus and oxygen. It has the empirical chemical formula {{chem2|P2O4}}. Solid diphosphorus tetroxide (also referred to as phosphorus(III,V)-oxide) consists of variable mixtures of the mixed-valence oxides P4O7, P4O8 and P4O9.[1][2][3]
Preparation
Phosphorus tetroxide is obtainable by thermal decomposition of phosphorus trioxide:
Phosphorus trioxide disproportionates above 210°C to form phosphorus and phosphorus tetroxide.
In addition, phosphorus trioxide can be converted into phosphorus tetroxide by controlled oxidation with oxygen in carbon tetrachloride solution.[4][5][6]
Careful reduction of phosphorus pentoxide with red phosphorus at 450-525 °C also produces the phosphorus tetroxide.
References
1. ^http://www.wiley.com/college/math/chem/cg/sales/voet.html.
2. ^{{cite book|author=Alberts B.|year=2002|title= Molecular Biology of the Cell, 4th Ed.|publisher= Garland Science|isbn=978-0-8153-4072-0|display-authors=etal}}
3. ^{{cite book|author=Voet D., Voet J. G.|title=Biochemistry, 3rd Ed.|publisher= Wiley|isbn=978-0-471-19350-0|date=2004-03-09}}
4. ^{{cite book|author=Atkins P., de Paula J.|year=2006|title=Physical chemistry, 8th Ed.|publisher= W. H. Freeman|place= San Francisco|isbn= 978-0-7167-8759-4}}
5. ^{{cite book |last1 = Petrucci |first1 = Ralph H. |last2 = Harwood |first2 = William S. |last3 = Herring |first3 = F. Geoffrey |date=2002 |title = General chemistry: principles and modern applications |edition=8th |location=Upper Saddle River, N.J |publisher=Prentice Hall |isbn = 978-0-13-014329-7 |lccn=2001032331 |oclc=46872308 |ref=harv }}
6. ^{{cite book|author=Laidler K. J.|year=1978|title=Physical chemistry with biological applications. Benjamin/Cummings|place= Menlo Park|isbn= 978-0-8053-5680-9}}
{{Phosphorus compounds}}2. ^{{cite book|author=Alberts B.|year=2002|title= Molecular Biology of the Cell, 4th Ed.|publisher= Garland Science|isbn=978-0-8153-4072-0|display-authors=etal}}
3. ^{{cite book|author=Voet D., Voet J. G.|title=Biochemistry, 3rd Ed.|publisher= Wiley|isbn=978-0-471-19350-0|date=2004-03-09}}
4. ^{{cite book|author=Atkins P., de Paula J.|year=2006|title=Physical chemistry, 8th Ed.|publisher= W. H. Freeman|place= San Francisco|isbn= 978-0-7167-8759-4}}
5. ^{{cite book |last1 = Petrucci |first1 = Ralph H. |last2 = Harwood |first2 = William S. |last3 = Herring |first3 = F. Geoffrey |date=2002 |title = General chemistry: principles and modern applications |edition=8th |location=Upper Saddle River, N.J |publisher=Prentice Hall |isbn = 978-0-13-014329-7 |lccn=2001032331 |oclc=46872308 |ref=harv }}
6. ^{{cite book|author=Laidler K. J.|year=1978|title=Physical chemistry with biological applications. Benjamin/Cummings|place= Menlo Park|isbn= 978-0-8053-5680-9}}
3 : Oxides|Inorganic phosphorus compounds|Solids
- Artemus L. Gates
- Artem Vasilyev
- Artem Vaskov
- Artem Vitalyevich Anisimov
- Artem Vodyakov
- Artem Voronkin
- Artem Wodjakov
- Artem Wodjakow
- Artem Yankovskiy
- Artem Yankovsky
- Artem Yarchuk
- Artemy Dzhidzhikhia
- Artem Yenin
- Artemyeva
- Artem Yevlyanov
- Artemy Ogarkov
- Artemy Petrovich Volynsky
- Artemy Troitsky
- Artem Yudin
- Artem Zabelin
- Artem Zagrebin
- Artem Zasyadvovk
- Artem Zhmurko
- Artem Łaguta
- Artena angulata