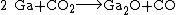- Production
- Properties
- References
| Watchedfields = changed
| verifiedrevid =
| Name = Gallium(I) oxide
| ImageFile =
| ImageName =
| OtherNames = gallium suboxide
digallium monoxide
|Section1={{Chembox Identifiers
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}}
| ChemSpiderID = 34990294
| StdInChI=1S/2Ga.O
| StdInChIKey = KXACIVYKDCQNQA-UHFFFAOYSA-N
| SMILES = O([Ga])[Ga]
| CASNo_Ref = {{cascite|correct|CAS}}
| CASNo = 12024-20-3
| PubChem = 16702109
| RTECS =
|Section2={{Chembox Properties
| Formula = Ga2O
| MolarMass = 155.445 g/mol[1]
| Appearance = brown powder[1]
| Density = 4.77 g/cm3[1]
| Solubility =
| MeltingPt = >800 °C
| MeltingPt_notes = (decomposes)
| MeltingPt_ref=[1]
| BoilingPt =
| SolubleOther =
| MagSus = -34·10−6 cm3/mol[2]
|Section5={{Chembox Thermochemistry
| DeltaHf = −356.2 kJ/mol[3]
| Entropy =
| DeltaGf =
| HeatCapacity =
}}
|Section7={{Chembox Hazards
| EUClass = not listed
}}
Gallium(I) oxide, digallium monoxide or gallium suboxide is an inorganic compound with the formula Ga2O.
Production
Gallium(I) oxide can be produced by reacting gallium(III) oxide with heated gallium in vacuum:[7]
It can also be obtained by reacting gallium with carbon dioxide in vacuum at 850 °C.[4]
Gallium(I) oxide is a by-product in the production of gallium arsenide wafers:[5][6]
Properties
Gallium(I) oxide is a brown-black diamagnetic solid which is resistant to further oxidation in dry air. It starts decomposing upon heating at temperatures above 500 °C, and the decomposition rate depends on the atmosphere (vacuum, inert gas, air).[7]
References
1. ^1 2 3 {{RubberBible92nd|page=4.64}}
2. ^{{RubberBible92nd|page=4.133}}
3. ^{{RubberBible92nd|page=5.12}}
4. ^{{cite book | author = Emeléus, H. J. and Sharpe, A. G. |volume=5| title = Advances in Inorganic Chemistry and Radiochemistry | publisher = Academic Press |isbn = 008057854-3 | year = 1963 | page = 94 }}
5. ^{{cite book | author = Siffert, Paul and Krimmel, Eberhard | title = Silicon: Evolution and Future of a Technology | publisher = Springer | isbn = 354040546-1 | year = 2004 | page = 439 }}
6. ^{{cite book | author = Chou, L. -J | title = Nanoscale One-dimensional Electronic and Photonic Devices (NODEPD) | publisher = The Electrochemical Society | isbn = 156677574-4 | year = 2007 |page= 47}}
7. ^1 {{cite book | author =Brauer, Georg |title=Handbuch der Präparativen Anorganischen Chemie|volume= 3|year=1975|isbn=3-432-02328-6|page=857}}
{{Gallium compounds}}{{Oxides}}{{oxygen compounds}}{{DEFAULTSORT:Gallium(I) oxide}}2. ^{{RubberBible92nd|page=4.133}}
3. ^{{RubberBible92nd|page=5.12}}
4. ^{{cite book | author = Emeléus, H. J. and Sharpe, A. G. |volume=5| title = Advances in Inorganic Chemistry and Radiochemistry | publisher = Academic Press |isbn = 008057854-3 | year = 1963 | page = 94 }}
5. ^{{cite book | author = Siffert, Paul and Krimmel, Eberhard | title = Silicon: Evolution and Future of a Technology | publisher = Springer | isbn = 354040546-1 | year = 2004 | page = 439 }}
6. ^{{cite book | author = Chou, L. -J | title = Nanoscale One-dimensional Electronic and Photonic Devices (NODEPD) | publisher = The Electrochemical Society | isbn = 156677574-4 | year = 2007 |page= 47}}
7. ^1 {{cite book | author =Brauer, Georg |title=Handbuch der Präparativen Anorganischen Chemie|volume= 3|year=1975|isbn=3-432-02328-6|page=857}}
2 : Oxides|Gallium compounds
- Matt Ramsden
- Matt Ramsey
- Matt Rapley
- Mattravile Soldiers' Settlement Public School
- Matt Ravlich
- Matt redman
- Matt Redmile
- Matt Reem
- Matt Reeves
- Matt reeves
- Matt Regan
- Matt Reilly
- Matt Reiswerg
- Matt (Resident Evil)
- Mattress.com
- Mattresses
- Mattress Factory
- Mattress mac
- Mattress Mack
- Mattress pads
- Mattress protector
- Mattress Protectors
- Mattress (rocket)
- Mattress size
- Mattress tag