- References
The Nickel ternary chalcogenides are a class of chemical compounds that contains nickel, a chalcogenide, and another element. Nickel forms a series of double nickel oxides with other elements, which may be termed "nickelates". These double nickel oxides are not listed on this page. There are also many well defined double compounds with sulfur, selenium and tellurium which are listed here.
| formula | name | colour | structure | production | references |
|---|---|---|---|---|---|
| NH4NiS5 | ammonium nickel sulphide | black | NH4 polysulfide+NiSO4 | [1][1] | |
| K2Ni3S4 | potassium nickel tetrasulfide | bronze yellow | Fddd a=10.023 b=26.074 c=5.704 | NiSO4 K2CO3 S | [1][2][3] |
| K2Ni11S10 | potassium nickel decasulfide | dark metallic green | NiO+KCNS | [4] J. Milbauer | |
| Na2Ni3S4 | sodium nickel tetrasulfide | dark yellow | NiSO4 Na2CO3 S | [4] R. Schneider | |
| KNi2S2 | potassium dinickel disulfide | orange yellow | Ni foil, S, K at 723K | [5][6] | |
| K2Ni3Se4 | potassium nickel tetraselenide | gold | Fddd a=10.468 b=26.496 c=5.995 | [7][11] | |
| KNi2Se2 | potassium dinickel diselenide | purple-red | I4/mmmtetragonal a=3.909, c=13.4142 | Ni foil, Se shot, K at 723K | [5][8] |
| CsNi2Se2 | caesium dinickel diselenide | tetragonal a=3.988, c=14.419 | heat elements | [9] | |
| TlNi2Se2 | Thallium dinickel diselenide | gold metallic | tetragonal | heat elements together in closed quartz tube | [10] |
| Rb2Ni3S4 | rubidium nickel tetrasulphide | metallic greenish gold | Fmmm orthorhombic a=9.901 Å, b=13.606 Å and c=5.861 Z=4 layered; ferromagnetic only after water immersion | [7][11] | |
| Rb2Ni3Se4 | rubidium nickel tetraselenide | golden metallic | Fddd orthorhombic a = 10.555 Å, b = 27.588 Å, c = 6.031 Å, Z = 8 layered; ferromagnetic only after water immersion | Rb2CO3 S Ni | [12] |
| Cs2Ni3S4 | cesium nickel tetrasulphide | greenish gold | Fmmm a=10.038 b=14.552 c=5.934 | [11][12] | |
| Cs2Ni3S4 | cesium nickel tetrasulphide | gold | Fmmm a=10.540 b=14.624 c=6.194 | [13] | |
| Cs0.9Ni3.1Se3 | Hexagonal P63/m a = 9.26301(4) Å and c = 4.34272(2) Å | quasi-one-dimensional electric conductor | [14] | ||
| BaNi4S5 | Barium nickel pentasulfide | bronze yellow | NiSO4 K2CO3 S | [4] R. Schneider; I. and L. Bellucci | |
| Pb2Ni3S2 | lead nickel disulfide | melt 790° | PbS Ni | [15] W Guertler; W Guertler H Schack | |
| (Ni,Fe)9S8 | Pentlandite | bronze yellow melt 870 | [15] T. Scheerer | ||
| Fe2Ni2S4 | ferrous nickel tetrasulfide | melt 840 | [16] K Bornemann | ||
| Fe2Ni2S3 | ferrous nickel trisulfide | stable over 575°, melt 886, | [17] K Bornemann | ||
| Fe3Ni4S5 | ferrous nickel pentasulfide | below 575 | [17] K Bornemann | ||
| Fe4Ni2S5 | [17] K Bornemann | ||||
| Fe2Ni3S4 | [17] K Bornemann | ||||
| Fe3Ni4S5 | [17] K Bornemann | ||||
| Fe2Ni2S3 | [17] K Bornemann | ||||
| FeNi2S4 | Violarite | dark violet grey | mineral oxidate | ||
| Ni3Sn2S2 | [18] | ||||
| Ni3Bi2S2 | superconducting | [18] | |||
| Ni3Bi2Se2 | superconducting | [18] | |||
| NiSnS3 | nickel thiostannate | greenish black | orthorhombic a=6.88 b=7.89 c=11.95 Z=8 V=644 | NiCl2 + SnS2 | [19] |
| NiGeS33 | nickelselenogermanate | [20] | |||
| Ta2NiS5 | Orthorhombic | [21] | |||
| Ta2NiSe5 | monoclinic β=90.53 | [21] | |||
| Ta2Ni2Te4 | [22] | ||||
| Ta2Ni3Te5 | [22] |
References
1. ^{{cite journal |last1=Ephraim |first1=Fritz |title=Über Nickelsulfid |journal=Berichte der deutschen chemischen Gesellschaft (A and B Series) |date=17 September 1923 |volume=56 |issue=8 |pages=1885–1886 |doi=10.1002/cber.19230560825 |language=de}}
2. ^{{cite journal |last1=Bronger |first1=W. |last2=Eyck |first2=J. |last3=Rüdorff |first3=W. |last4=Stöussel |first4=A. |title=Über Thio- und Selenoniccolate und -palladate der schweren Alkalimetalle |journal=Zeitschrift für anorganische und allgemeine Chemie |date=July 1970 |language=de |volume=375 |issue=1 |pages=1–7 |doi=10.1002/zaac.19703750102}}
3. ^{{cite journal |last1=Schneider |first1=R. |title=Ueber neue Schwefelsalze |journal=Annalen der Physik und Chemie |date=1874 |volume=227 |issue=3 |pages=437–450 |doi=10.1002/andp.18742270306|bibcode=1874AnP...227..437S }}
4. ^1 2 3 4 Mellor p443
5. ^1 {{cite journal |last1=Hlukhyy |first1=Viktor |last2=Trots |first2=Dmytro |last3=Fässler |first3=Thomas F. |title=First-Order Phase Transition in BaNi2Ge2 and the Influence of the Valence Electron Count on Distortion of the Structure Type |journal=Inorganic Chemistry |volume=56 |issue=3 |pages=1173 |date=13 January 2017 |doi=10.1021/acs.inorgchem.6b02190|pmid=28085271 }}
Structure Type |journal=Inorganic Chemistry |volume=56 |issue=3 |pages=1173 |date=13 January 2017 |doi=10.1021/acs.inorgchem.6b02190|pmid=28085271 }}
6. ^{{cite journal |last1=Neilson |first1=James R. |last2=McQueen |first2=Tyrel M. |last3=Llobet |first3=Anna |last4=Wen |first4=Jiajia |last5=Suchomel |first5=Matthew R. |title=Charge density wave fluctuations, heavy electrons, and superconductivity in |journal=Physical Review B |date=24 January 2013 |volume=87 |issue=4 |pages=045124 |doi=10.1103/PhysRevB.87.045124|bibcode=2013PhRvB..87d5124N |arxiv=1212.4744 }}
|journal=Physical Review B |date=24 January 2013 |volume=87 |issue=4 |pages=045124 |doi=10.1103/PhysRevB.87.045124|bibcode=2013PhRvB..87d5124N |arxiv=1212.4744 }}
7. ^1 {{cite journal |last1=Bronger |first1=W. |last2=Eyck |first2=J. |last3=Rüdorff |first3=W. |last4=Stöussel |first4=A. |title=Über Thio- und Selenoniccolate und -palladate der schweren Alkalimetalle |journal=Zeitschrift für anorganische und allgemeine Chemie |date=July 1970 |volume=375 |issue=1 |pages=1–7 |doi=10.1002/zaac.19703750102}}
8. ^{{cite journal |last1=Neilson |first1=James R. |last2=McQueen |first2=Tyrel M. |title=Bonding, Ion Mobility, and Rate-Limiting Steps in Deintercalation Reactions with -type
-type  |journal=Journal of the American Chemical Society |date=9 May 2012 |volume=134 |issue=18 |pages=7750–7757 |doi=10.1021/ja212012k}}
|journal=Journal of the American Chemical Society |date=9 May 2012 |volume=134 |issue=18 |pages=7750–7757 |doi=10.1021/ja212012k}}
9. ^{{cite journal |last1=Huimin Chen |last2=Yang |first2=Jinhu |last3=Cao |first3=Chao |last4=Li |first4=Lin |last5=Su |first5=Qiping |last6=Chen |first6=Bin |last7=Wang |first7=Hangdong |last8=Mao |first8=Qianhui |last9=Xu |first9=Binjie |last10=Du |first10=Jianhua |last11=Minghu Fang |title=Superconductivity in a new layered nickel selenide |journal=Superconductor Science and Technology |date=1 April 2016 |volume=29 |issue=4 |pages=045008 |doi=10.1088/0953-2048/29/4/045008|bibcode=2016SuScT..29d5008C |arxiv=1508.05167 }}
|journal=Superconductor Science and Technology |date=1 April 2016 |volume=29 |issue=4 |pages=045008 |doi=10.1088/0953-2048/29/4/045008|bibcode=2016SuScT..29d5008C |arxiv=1508.05167 }}
10. ^{{cite journal |last1=Wang |first1=Hangdong |last2=Dong |first2=Chiheng |last3=Mao |first3=Qianhui |last4=Khan |first4=Rajwali |last5=Zhou |first5=Xi |last6=Li |first6=Chenxia |last7=Chen |first7=Bin |last8=Yang |first8=Jinhu |last9=Su |first9=Qiping |last10=Fang |first10=Minghu |title=Multiband Superconductivity of Heavy Electrons in a Single Crystal |journal=Physical Review Letters |date=12 November 2013 |volume=111 |issue=20 |pages=207001 |doi=10.1103/PhysRevLett.111.207001|pmid=24289702 |bibcode=2013PhRvL.111t7001W |arxiv=1303.4658 }}
Single Crystal |journal=Physical Review Letters |date=12 November 2013 |volume=111 |issue=20 |pages=207001 |doi=10.1103/PhysRevLett.111.207001|pmid=24289702 |bibcode=2013PhRvL.111t7001W |arxiv=1303.4658 }}
11. ^{{cite journal |last1=Hasegawa |first1=Takumi |last2=Inui |first2=Mitsutaka |last3=Hondou |first3=Katsuhiro |last4=Fujiwara |first4=Yishihiro |last5=Kato |first5=Tetsuya |last6=Iio |first6=Katsunori |title=Raman spectroscopy on ternary transition metal chalcogenide |journal=Journal of Alloys and Compounds |date=February 2004 |volume=364 |issue=1–2 |pages=199–207 |doi=10.1016/S0925-8388(03)00503-6}}
|journal=Journal of Alloys and Compounds |date=February 2004 |volume=364 |issue=1–2 |pages=199–207 |doi=10.1016/S0925-8388(03)00503-6}}
12. ^1 {{cite journal |last1=Bronger |first1=Welf |last2=Rennau |first2=R. M. |last3=Schmitz |first3=D. |title=Darstellung und Kristallstruktur von |journal=Zeitschrift für anorganische und allgemeine Chemie |date=April 1996 |volume=622 |issue=4 |pages=627–629 |doi=10.1002/zaac.19966220409}}
|journal=Zeitschrift für anorganische und allgemeine Chemie |date=April 1996 |volume=622 |issue=4 |pages=627–629 |doi=10.1002/zaac.19966220409}}
13. ^1 2 {{cite journal |last1=Bronger |first1=W. |last2=Rennau |first2=R. |last3=Schmitz |first3=D. |title=Schichtstrukturen ternärer Chalkogenide (A ? K, Rb, Cs; M ? Ni, Pd, Pt; X ? S, Se) |journal=Zeitschrift für anorganische und allgemeine Chemie |date=May 1991 |volume=597 |issue=1 |pages=27–32 |doi=10.1002/zaac.19915970105}}
(A ? K, Rb, Cs; M ? Ni, Pd, Pt; X ? S, Se) |journal=Zeitschrift für anorganische und allgemeine Chemie |date=May 1991 |volume=597 |issue=1 |pages=27–32 |doi=10.1002/zaac.19915970105}}
14. ^{{cite journal|last1=Sun|first1=Fan|last2=Guo|first2=Zhongnan|last3=Liu|first3=Ning|last4=Wu|first4=Dan|last5=Lin|first5=Jiawei|last6=Cheng|first6=Erjian|last7=Ying|first7=Tianping|last8=Li|first8=Shiyan|last9=Yuan|first9=Wenxia|title=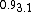 : A Ni-Based Quasi-One-Dimensional Conductor with Spin-Glass Behavior|journal=Inorganic Chemistry|date=16 March 2018|doi=10.1021/acs.inorgchem.7b03143|volume=57|pages=3798–3804}}
: A Ni-Based Quasi-One-Dimensional Conductor with Spin-Glass Behavior|journal=Inorganic Chemistry|date=16 March 2018|doi=10.1021/acs.inorgchem.7b03143|volume=57|pages=3798–3804}}
15. ^1 Mellor p444
16. ^Mellor p445
17. ^1 2 3 4 5 Mellor p446
18. ^1 2 {{cite journal |last1=Rommel |first1=Stefan Michael |last2=Krach |first2=Alexander |last3=Peter |first3=Philipp |last4=Weihrich |first4=Richard |title=Conversion Reactions of Solids: From a Surprising Three-Step Mechanism towards Directed Product Formation |journal=Chemistry |date=25 April 2016 |volume=22 |issue=18 |pages=6333–6339 |doi=10.1002/chem.201505209}}
19. ^{{cite journal |last1=Mamedova |first1=N. A. |last2=Ragimov |first2=S. S. |last3=Sadykhov |first3=F. M. |last4=Aliev |first4=I. I. |title=Preparation and physicochemical study of nickel(II) thiostannate in the system |journal=Russian Journal of Inorganic Chemistry |date=17 February 2012 |volume=57 |issue=2 |pages=160–162 |doi=10.1134/S0036023612020179}}
system |journal=Russian Journal of Inorganic Chemistry |date=17 February 2012 |volume=57 |issue=2 |pages=160–162 |doi=10.1134/S0036023612020179}}
20. ^{{cite book |last1=Popescu |first1=M. A. |title=Non-Crystalline Chalcogenicides |publisher=Springer Science & Business Media |isbn=9781402003592 |page=73 |url=https://books.google.com/books?id=6RgQf8cPPbIC&pg=PA73 |language=en|date=2001-11-30 }}
21. ^1 {{cite journal |last1=Di Salvo |first1=F.J. |last2=Chen |first2=C.H. |last3=Fleming |first3=R.M. |last4=Waszczak |first4=J.V. |last5=Dunn |first5=R.G. |last6=Sunshine |first6=S.A. |last7=Ibers |first7=James A. |title=Physical and structural properties of the new layered compounds and
and  |journal=Journal of the Less Common Metals |date=February 1986 |volume=116 |issue=1 |pages=51–61 |doi=10.1016/0022-5088(86)90216-X}}
|journal=Journal of the Less Common Metals |date=February 1986 |volume=116 |issue=1 |pages=51–61 |doi=10.1016/0022-5088(86)90216-X}}
22. ^1 {{cite journal |last1=Tremel |first1=Wolfgang |title=Isolated and Condensed Ta2Ni2 Clusters in the Layered Tellurides and
and  |journal=Angewandte Chemie International Edition in English |date=July 1991 |volume=30 |issue=7 |pages=840–843 |doi=10.1002/anie.199108401}}
|journal=Angewandte Chemie International Edition in English |date=July 1991 |volume=30 |issue=7 |pages=840–843 |doi=10.1002/anie.199108401}}
2. ^{{cite journal |last1=Bronger |first1=W. |last2=Eyck |first2=J. |last3=Rüdorff |first3=W. |last4=Stöussel |first4=A. |title=Über Thio- und Selenoniccolate und -palladate der schweren Alkalimetalle |journal=Zeitschrift für anorganische und allgemeine Chemie |date=July 1970 |language=de |volume=375 |issue=1 |pages=1–7 |doi=10.1002/zaac.19703750102}}
3. ^{{cite journal |last1=Schneider |first1=R. |title=Ueber neue Schwefelsalze |journal=Annalen der Physik und Chemie |date=1874 |volume=227 |issue=3 |pages=437–450 |doi=10.1002/andp.18742270306|bibcode=1874AnP...227..437S }}
4. ^1 2 3 4 Mellor p443
5. ^1 {{cite journal |last1=Hlukhyy |first1=Viktor |last2=Trots |first2=Dmytro |last3=Fässler |first3=Thomas F. |title=First-Order Phase Transition in BaNi2Ge2 and the Influence of the Valence Electron Count on Distortion of the
 Structure Type |journal=Inorganic Chemistry |volume=56 |issue=3 |pages=1173 |date=13 January 2017 |doi=10.1021/acs.inorgchem.6b02190|pmid=28085271 }}
Structure Type |journal=Inorganic Chemistry |volume=56 |issue=3 |pages=1173 |date=13 January 2017 |doi=10.1021/acs.inorgchem.6b02190|pmid=28085271 }}6. ^{{cite journal |last1=Neilson |first1=James R. |last2=McQueen |first2=Tyrel M. |last3=Llobet |first3=Anna |last4=Wen |first4=Jiajia |last5=Suchomel |first5=Matthew R. |title=Charge density wave fluctuations, heavy electrons, and superconductivity in
 |journal=Physical Review B |date=24 January 2013 |volume=87 |issue=4 |pages=045124 |doi=10.1103/PhysRevB.87.045124|bibcode=2013PhRvB..87d5124N |arxiv=1212.4744 }}
|journal=Physical Review B |date=24 January 2013 |volume=87 |issue=4 |pages=045124 |doi=10.1103/PhysRevB.87.045124|bibcode=2013PhRvB..87d5124N |arxiv=1212.4744 }}7. ^1 {{cite journal |last1=Bronger |first1=W. |last2=Eyck |first2=J. |last3=Rüdorff |first3=W. |last4=Stöussel |first4=A. |title=Über Thio- und Selenoniccolate und -palladate der schweren Alkalimetalle |journal=Zeitschrift für anorganische und allgemeine Chemie |date=July 1970 |volume=375 |issue=1 |pages=1–7 |doi=10.1002/zaac.19703750102}}
8. ^{{cite journal |last1=Neilson |first1=James R. |last2=McQueen |first2=Tyrel M. |title=Bonding, Ion Mobility, and Rate-Limiting Steps in Deintercalation Reactions with
 -type
-type  |journal=Journal of the American Chemical Society |date=9 May 2012 |volume=134 |issue=18 |pages=7750–7757 |doi=10.1021/ja212012k}}
|journal=Journal of the American Chemical Society |date=9 May 2012 |volume=134 |issue=18 |pages=7750–7757 |doi=10.1021/ja212012k}}9. ^{{cite journal |last1=Huimin Chen |last2=Yang |first2=Jinhu |last3=Cao |first3=Chao |last4=Li |first4=Lin |last5=Su |first5=Qiping |last6=Chen |first6=Bin |last7=Wang |first7=Hangdong |last8=Mao |first8=Qianhui |last9=Xu |first9=Binjie |last10=Du |first10=Jianhua |last11=Minghu Fang |title=Superconductivity in a new layered nickel selenide
 |journal=Superconductor Science and Technology |date=1 April 2016 |volume=29 |issue=4 |pages=045008 |doi=10.1088/0953-2048/29/4/045008|bibcode=2016SuScT..29d5008C |arxiv=1508.05167 }}
|journal=Superconductor Science and Technology |date=1 April 2016 |volume=29 |issue=4 |pages=045008 |doi=10.1088/0953-2048/29/4/045008|bibcode=2016SuScT..29d5008C |arxiv=1508.05167 }}10. ^{{cite journal |last1=Wang |first1=Hangdong |last2=Dong |first2=Chiheng |last3=Mao |first3=Qianhui |last4=Khan |first4=Rajwali |last5=Zhou |first5=Xi |last6=Li |first6=Chenxia |last7=Chen |first7=Bin |last8=Yang |first8=Jinhu |last9=Su |first9=Qiping |last10=Fang |first10=Minghu |title=Multiband Superconductivity of Heavy Electrons in a
 Single Crystal |journal=Physical Review Letters |date=12 November 2013 |volume=111 |issue=20 |pages=207001 |doi=10.1103/PhysRevLett.111.207001|pmid=24289702 |bibcode=2013PhRvL.111t7001W |arxiv=1303.4658 }}
Single Crystal |journal=Physical Review Letters |date=12 November 2013 |volume=111 |issue=20 |pages=207001 |doi=10.1103/PhysRevLett.111.207001|pmid=24289702 |bibcode=2013PhRvL.111t7001W |arxiv=1303.4658 }}11. ^{{cite journal |last1=Hasegawa |first1=Takumi |last2=Inui |first2=Mitsutaka |last3=Hondou |first3=Katsuhiro |last4=Fujiwara |first4=Yishihiro |last5=Kato |first5=Tetsuya |last6=Iio |first6=Katsunori |title=Raman spectroscopy on ternary transition metal chalcogenide
 |journal=Journal of Alloys and Compounds |date=February 2004 |volume=364 |issue=1–2 |pages=199–207 |doi=10.1016/S0925-8388(03)00503-6}}
|journal=Journal of Alloys and Compounds |date=February 2004 |volume=364 |issue=1–2 |pages=199–207 |doi=10.1016/S0925-8388(03)00503-6}}12. ^1 {{cite journal |last1=Bronger |first1=Welf |last2=Rennau |first2=R. M. |last3=Schmitz |first3=D. |title=Darstellung und Kristallstruktur von
 |journal=Zeitschrift für anorganische und allgemeine Chemie |date=April 1996 |volume=622 |issue=4 |pages=627–629 |doi=10.1002/zaac.19966220409}}
|journal=Zeitschrift für anorganische und allgemeine Chemie |date=April 1996 |volume=622 |issue=4 |pages=627–629 |doi=10.1002/zaac.19966220409}}13. ^1 2 {{cite journal |last1=Bronger |first1=W. |last2=Rennau |first2=R. |last3=Schmitz |first3=D. |title=Schichtstrukturen ternärer Chalkogenide
 (A ? K, Rb, Cs; M ? Ni, Pd, Pt; X ? S, Se) |journal=Zeitschrift für anorganische und allgemeine Chemie |date=May 1991 |volume=597 |issue=1 |pages=27–32 |doi=10.1002/zaac.19915970105}}
(A ? K, Rb, Cs; M ? Ni, Pd, Pt; X ? S, Se) |journal=Zeitschrift für anorganische und allgemeine Chemie |date=May 1991 |volume=597 |issue=1 |pages=27–32 |doi=10.1002/zaac.19915970105}}14. ^{{cite journal|last1=Sun|first1=Fan|last2=Guo|first2=Zhongnan|last3=Liu|first3=Ning|last4=Wu|first4=Dan|last5=Lin|first5=Jiawei|last6=Cheng|first6=Erjian|last7=Ying|first7=Tianping|last8=Li|first8=Shiyan|last9=Yuan|first9=Wenxia|title=
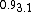 : A Ni-Based Quasi-One-Dimensional Conductor with Spin-Glass Behavior|journal=Inorganic Chemistry|date=16 March 2018|doi=10.1021/acs.inorgchem.7b03143|volume=57|pages=3798–3804}}
: A Ni-Based Quasi-One-Dimensional Conductor with Spin-Glass Behavior|journal=Inorganic Chemistry|date=16 March 2018|doi=10.1021/acs.inorgchem.7b03143|volume=57|pages=3798–3804}}15. ^1 Mellor p444
16. ^Mellor p445
17. ^1 2 3 4 5 Mellor p446
18. ^1 2 {{cite journal |last1=Rommel |first1=Stefan Michael |last2=Krach |first2=Alexander |last3=Peter |first3=Philipp |last4=Weihrich |first4=Richard |title=Conversion Reactions of Solids: From a Surprising Three-Step Mechanism towards Directed Product Formation |journal=Chemistry |date=25 April 2016 |volume=22 |issue=18 |pages=6333–6339 |doi=10.1002/chem.201505209}}
19. ^{{cite journal |last1=Mamedova |first1=N. A. |last2=Ragimov |first2=S. S. |last3=Sadykhov |first3=F. M. |last4=Aliev |first4=I. I. |title=Preparation and physicochemical study of nickel(II) thiostannate in the
 system |journal=Russian Journal of Inorganic Chemistry |date=17 February 2012 |volume=57 |issue=2 |pages=160–162 |doi=10.1134/S0036023612020179}}
system |journal=Russian Journal of Inorganic Chemistry |date=17 February 2012 |volume=57 |issue=2 |pages=160–162 |doi=10.1134/S0036023612020179}}20. ^{{cite book |last1=Popescu |first1=M. A. |title=Non-Crystalline Chalcogenicides |publisher=Springer Science & Business Media |isbn=9781402003592 |page=73 |url=https://books.google.com/books?id=6RgQf8cPPbIC&pg=PA73 |language=en|date=2001-11-30 }}
21. ^1 {{cite journal |last1=Di Salvo |first1=F.J. |last2=Chen |first2=C.H. |last3=Fleming |first3=R.M. |last4=Waszczak |first4=J.V. |last5=Dunn |first5=R.G. |last6=Sunshine |first6=S.A. |last7=Ibers |first7=James A. |title=Physical and structural properties of the new layered compounds
 and
and  |journal=Journal of the Less Common Metals |date=February 1986 |volume=116 |issue=1 |pages=51–61 |doi=10.1016/0022-5088(86)90216-X}}
|journal=Journal of the Less Common Metals |date=February 1986 |volume=116 |issue=1 |pages=51–61 |doi=10.1016/0022-5088(86)90216-X}}22. ^1 {{cite journal |last1=Tremel |first1=Wolfgang |title=Isolated and Condensed Ta2Ni2 Clusters in the Layered Tellurides
 and
and  |journal=Angewandte Chemie International Edition in English |date=July 1991 |volume=30 |issue=7 |pages=840–843 |doi=10.1002/anie.199108401}}
|journal=Angewandte Chemie International Edition in English |date=July 1991 |volume=30 |issue=7 |pages=840–843 |doi=10.1002/anie.199108401}} 2 : Nickel compounds|Chalcogenides
- Radiohjälpen
- Radio Homogenization
- Radio homogenization
- Radio HornAfrik
- Radiohuset
- Radiohuset (Stockholm)
- Radio-i
- Radio Iasi
- Radio Iaşi
- Radio Ice Cerenkov Experiment
- Radio imaging
- Radio Impuls (Czech Republic)
- Radio in a box
- Radio in Austria
- Radio in Bahrain
- Radio in Bulgaria
- Radio in Canada
- Radio in Colombia
- Radio in Croatia
- Radio India
- Radio India Ltd.
- Radio Indigo
- Radio Indigo FM
- Radio Industri
- Radio in Estonia