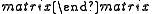- Career
- Major Contributions
- Publications
- See also
- References
- External links
|name = Tamejiro Hiyama
|birth_date = {{Birth date and age|1946|08|24|mf=yes}}
|birth_place = Ibaraki, Osaka, Japan
|nationality = Japan
|field = Organic chemistry Organometallic chemistry
|work_institutions = Chuo University
|alma_mater = Kyoto University
|doctoral_advisor = Hitoshi Nozaki
|known_for = Nozaki-Hiyama-Kishi reaction Hiyama coupling
|prizes = {{Plainlist|
- The Chemical Society of Japan Award for Young Chemists (1980)
- The Bulletin of the Chemical Society of Japan Award (2001)
- The Japan Liquid Crystal Society Award (2004)
- The Society of Synthetic Organic Chemistry of Japan Award(2007)
- The Chemical Society of Japan Award (2008)
- Boehringer-Ingelheim Lectureship (2010)
- The Bulletin of the Chemical Society of Japan Award (2010)
- Humboldt Research Award (2012)}}
| website = {{URL|http://www.chem.chuo-u.ac.jp/~omega300/index.html}}
Tamejiro Hiyama (born August 24, 1946) is a Japanese organic chemist. He is best known for his work in developing the Nozaki-Hiyama-Kishi reaction and the Hiyama coupling. He is currently a professor at the Chuo University Research and Development Initiative, and a Professor Emeritus of Kyoto University.
Career
Hiyama received his Bachelor of Engineering (1969) and Master of Engineering (1971) from Kyoto University. He dropped out of the doctorate track in 1972, and subsequently started working as an assistant for Hitoshi Nozaki at Kyoto University. In 1975, he obtained his doctoral degree, and during 1975-1976 conducted postdoctoral research with Yoshito Kishi at Harvard University. In 1981, he started working at the Sagami Chemical Research Center, and became a principal investigator in 1983, and then chief laboratory manager in 1988.[1]
In 1992, he re-entered the world of academia at the Tokyo Institute of Technology as a professor of the Research Laboratory of Resources Utilization. He then returned to Kyoto University in 1997 as a professor of engineering, until 2010 when he transferred to Chuo University, where he currently holds tenure.
His current research focuses on C-H activation[2] and cross-coupling reactions.[3] In particular, he is interested in ortho and benzylic C-H activation, and C-C, C-N, and C-Si bond formation via cross-coupling with organosilicon reagents.[4][5]
In his spare time, he enjoys listening to classical music. His favorite way of spending a holiday is “cleaning [his] small garden by picking out weeds one by one”, which is “good psychological training for a Buddhist priest”.[6]
Major Contributions
Hiyama is best known for developing:
- The Nozaki-Hiyama-Kishi reaction (NHK reaction) is a nickel/chromium mediated cross-coupling reaction between an allyl, vinyl or aryl halide and an aldehyde to form an alcohol upon aqueous workup.
It was originally discovered in 1977, where Hiyama and Nozaki reported a chemospecific synthesis of homoallyl alcohols from an aldehyde and allyl halide using chromium(II) chloride.[7]
In 1983, Hiyama and Nozaki published another paper extending the scope of the reaction to include aryl and vinyl halides.[8]
In 1986, Nozaki and Kishi independently discovered that the reaction depended on the nickel impurities in the chromium(II) chloride salt.[9][10] Since then, nickel(II) chloride has been used as a co-catalyst.[11]
The NHK reaction demonstrates high chemoselectivity towards aldehydes, as it tolerates a range of functional groups,[12] and has been used on the process scale.[13]
- The Hiyama coupling is a palladium-catalyzed cross-coupling reaction between aryl, alkenyl or alkyl halides and an organosilicon compound to form a C-C bond.
-
R : Aryl, Alkenyl or Alkynyl -
R' : Aryl, Alkenyl, Alkynyl or Alkyl -
R : Cl, F or Alkyl -
X : Cl, Br, I or OTf
Hiyama developed this reaction in 1988.[14][15] He says he developed this method in order to overcome the shortcomings of Grignard reagents. While Grignard reagents are powerful, Hiyama says, they can be hard to use in total synthesis as they are not as tolerant of other functional groups.[5]
Publications
He has published over 400 papers and 25 books over the course of his career.[16]
Notable publications include:
- Tamejiro Hiyama and Koichiro Oshima, “有機合成化学” [Organic Synthetic Chemistry], Tokyo Kagaku Dojin, 2012, {{ISBN|978-4807907601}}
- G. S. Zweifel, M. H. Nantz, Tamejiro Hiyama, “最新有機合成法 設計と戦略 – Modern Organic Synthesis: An Introduction”, Kagaku Dojin, 2009, {{ISBN|978-4759811742}}
- Tamejiro Hiyama, Kyoko Nozaki, “有機合成のための触媒反応” [Catalytic Reactions for Organic Synthetic Chemistry], Tokyo Kagaku Dojin, 2004, {{ISBN|978-4807905867}}
- Tamejiro Hiyama, edited by Hisashi Yamamoto, “Organofluorine Compounds: Chemistry and Applications”, Springer, 2000, {{ISBN|978-3-662-04164-2}}
See also
- Nozaki-Hiyama-Kishi reaction
- Hiyama coupling
References
2. ^{{cite journal| last1 = Minami | first1 = Y. | last2 = Hiyama | first2 = T. | year = 2016 | title = Synthetic Transformations through Alkynoxy-Palladium Interactions and C-H Activation | url = | journal = Acc. Chem. Res. | volume = 49 | issue = 1| pages = 67–77 | doi = 10.1021/acs.accounts.5b00414}}
3. ^{{cite journal| last1 = Komiyama | first1 = T. | last2 = Minami | first2 = Y. | last3 = Hiyama | first3 = T. | year = 2017 | title = Recent Advances in Transition-Metal-Catalyzed Synthetic Transformations of Organosilicon Reagents | url = | journal = ACS Catal. | volume = 7 | issue = 1| pages = 631–651 | doi = 10.1021/acscatal.6b02374}}
4. ^Hiyama Lab Website--Research {{Webarchive|url=https://web.archive.org/web/20170223101506/http://www.chem.chuo-u.ac.jp/~omega300/about.html |date=February 23, 2017 }}
5. ^1 Hiyama Interview {{webarchive|url=http://webcache.googleusercontent.com/search?q=cache:pb7_lxbgwZcJ:www.yomiuri.co.jp/adv/chuo/research/20120607.html+&cd=10&hl=en&ct=clnk&gl=us|date=April 13, 2017}}
6. ^{{cite journal | last1 = Hiyama | first1 = T. | year = 2017 | title = Author Profile: Tamejiro Hiyama | url = | journal = Angew. Chem. Int. Ed. | volume = 56 | issue = 9| pages = 2242–2244 | doi = 10.1002/anie.201608230}}
7. ^{{cite journal | last1 = Okude | first1 = Y. | last2 = Hirano | first2 = S. | last3 = Hiyama | first3 = T. | last4 = Nozaki | first4 = H. | year = 1977 | title = Grignard-type carbonyl addition of allyl halides by means of chromous salt. A chemospecific synthesis of homoallyl alcohols| url = | journal = J. Am. Chem. Soc. | volume = 99 | issue = 9| pages = 3179–3181 | doi = 10.1021/ja00451a061}}
8. ^{{cite journal | last1 = Takai | first1 = K. | last2 = Kimura | first2 = K. | last3 = Kuroda | first3 = T. | last4 = Hiyama | first4 = T. | last5 = Nozaki | first5 = H. | year = 1983 | title = Selective grignard-type carbonyl addition of alkenyl halides mediated by chromium(II) chloride| url = | journal = Tett. Lett. | volume = 24 | issue = 47| pages = 5281–5284 | doi = 10.1016/S0040-4039(00_88417-8}}
9. ^{{cite journal | last1 = Takai | first1 = K. | last2 = Tagashira | first2 = M. | last3 = Kuroda | first3 = T. | last4 = Oshima| first4 = K. | last5 = Utimoto | first5 = K. | last6 = Nozaki | first6 = H. | year = 1986 | title = Reactions of alkenylchromium reagents prepared from alkenyl trifluoromethanesulfonates (triflates) with chromium(II) chloride under nickel catalysis | url = | journal = J. Am. Chem. Soc. | volume = 108| issue = 19| pages = 6048–6050 | doi = 10.1021/ja00279a068}}
10. ^{{cite journal | last1 = Haolun | first1 = J. | last2 = Uenishi | first2 = J. | last3 = Christ| first3 = W.J. | last4 = Kishi| first4 = Y. | year = 1986 | title = Catalytic effect of nickel(II) chloride and palladium(II) acetate on chromium(II)-mediated coupling reaction of iodo olefins with aldehydes | url = | journal = J. Am. Chem. Soc. | volume = 108| issue = 18| pages = 5644–5646 | doi = 10.1021/ja00278a057}}
11. ^{{cite journal | last1 = Thomé | first1 = I. | last2 = Nijs | first2 = A. | last3 = Bolm | first3 = C. | year = 2012 | title = Trace metal impurities in catalysis | url = | journal = Chem. Soc. Rev. | volume = 41| pages = 979–987 | doi = 10.1039/c2cs15249e}}
12. ^{{cite journal | year = 1994 | title = Chromium(II)-based methods for carbon-carbon bond formation | url = | journal = J. Organomet. Chem. | volume = 476|issue = 1| pages = 1–5 | doi = 10.1016/0022-328X(94)84132-2}}
13. ^{{cite journal | year = 2013 | title = Process Development of Halaven®: Synthesis of the C14-C35 Fragment via Iterative Nozaki-Hiyama-Kishi Reaction-Williamson Ether Cyclization | url = | journal = Synlett | volume = 24|issue = 3| pages = 327–332 | doi = 10.1039/c2cs15249e}}
14. ^{{cite journal | last1 = Hatanaka| first1 = Y. | last2 = Hiyama | first2 = T. | year = 1988 | title = Cross-coupling of organosilanes with organic halides mediated by a palladium catalyst and tris(diethylamino) sulfonium difluorotrimethylsilicate| url = | journal = J. Org. Chem. | volume = 53 | issue = 4| pages = 918–920 | doi = 10.1021/jo00239a056}}
15. ^{{cite journal |last1 = Hiyama | first1 = T. | year = 2002| title = How I came across the silicon-based cross-coupling reaction| url = | journal = J. Organomet. Chem. | volume = 653 | issue = 1-2| pages = 58–61 | doi = 10.1016/S0022-328X(02)01157-9}}
16. ^Hiyama Lab Website-Publications {{Webarchive|url=https://web.archive.org/web/20170421100418/http://www.chem.chuo-u.ac.jp/~omega300/publication2010-.html |date=April 21, 2017 }}
External links
- Hiyama Lab Website
- Shelter (film)
- Shelter for Life
- Shelter From The Ash
- Shelter from the Ash
- Shelter from the storm
- Shelter home
- Sheltering Arms Early Education and Family Centers
- Shelter in place
- Shelter in Place
- Shelter in the Rain
- Shelter Island (Alaska)
- Shelter Island Country Club
- Shelter Island (disambiguation)
- Shelter Island Heights Historic District
- Shelter Island Heights, NY
- Shelter Island (How I Met Your Mother)
- Shelter Island NY
- Shelter Island, NY
- Shelter Islands
- Shelter Island, San Diego
- Shelter Island, San Diego, California
- Shelter Island Town, New York Police Department
- Shelter Island Town Police Department
- Shelter Island (Western Australia)
- Shelter Island Windmill