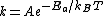- Worked example
- See also
In chemical kinetics, an Arrhenius plot displays the logarithm of a reaction rate constant, ( , ordinate axis) plotted against inverse temperature (
, ordinate axis) plotted against inverse temperature ( , abscissa). Arrhenius plots are often used to analyze the effect of temperature on the rates of chemical reactions. For a single rate-limited thermally activated process, an Arrhenius plot gives a straight line, from which the activation energy and the pre-exponential factor can both be determined.
, abscissa). Arrhenius plots are often used to analyze the effect of temperature on the rates of chemical reactions. For a single rate-limited thermally activated process, an Arrhenius plot gives a straight line, from which the activation energy and the pre-exponential factor can both be determined.
| Example: Nitrogen dioxide decay |
|---|
The Arrhenius equation can be given in the form:
or alternatively
The only difference is the energy units: the former form uses energy/mole, which is common
in chemistry, while the latter form uses energy directly, which is common in physics.
The different units are accounted for in using either the gas constant 
or the Boltzmann constant  .
.
The former form can be written equivalently as:
Where:
= Rate constant
= Pre-exponential factor
= Activation energy
= Gas constant
= Absolute temperature, K
When plotted in the manner described above, the value of the true y-intercept (at  ) will correspond to
) will correspond to  , and the slope of the line will be equal to
, and the slope of the line will be equal to  . The values of y-intercept and slope can be determined from the experimental points using simple linear regression with a spreadsheet.
. The values of y-intercept and slope can be determined from the experimental points using simple linear regression with a spreadsheet.
The pre-exponential factor, A, is an empirical constant of proportionality which has been estimated by various theories which take into account factors such as the frequency of collision between reacting particles, their relative orientation, and the entropy of activation.
The expression  represents the fraction of the molecules present in a gas which have energies equal to or in excess of activation energy at a particular temperature. In almost all practical cases,
represents the fraction of the molecules present in a gas which have energies equal to or in excess of activation energy at a particular temperature. In almost all practical cases,  , so that this fraction is very small and increases rapidly with T. In consequence, the reaction rate constant k increases rapidly with temperature T, as shown in the direct plot of k(T). (Mathematically, at very high temperatures so that
, so that this fraction is very small and increases rapidly with T. In consequence, the reaction rate constant k increases rapidly with temperature T, as shown in the direct plot of k(T). (Mathematically, at very high temperatures so that  , k would level off and approach A as a limit, but this case does not occur under practical conditions.)
, k would level off and approach A as a limit, but this case does not occur under practical conditions.)
Worked example
Based on the red "line of best fit" plotted in the graph given above:
Let y = ln(k[10−4 cm3 mol−1 s−1])
Let x = 1/T[K]
Points read from graph:
y = 4.1 at x = 0.0015
y = 2.2 at x = 0.00165
Slope of red line = (4.1 - 2.2) / (0.0015 - 0.00165) = -12,667
Intercept [y-value at x=0] of red line = 4.1 + (0.0015 x 12667) = 23.1
Inserting these values into the form above:
yields:
as shown in the plot at the right.
for:
k in 10−4 cm3 mol−1 s−1
T in K
Substituting for the quotient in the exponent of  :
:
-Ea / R = -12,667 K
approximate value for R = 8.31446 J K−1 mol−1
The activation energy of this reaction from these data is then:
Ea = R x 12,667 K = 105,300 J mol−1 = 105.3 kJ mol−1.
See also
- Arrhenius equation
- Eyring equation
- Polymer degradation
- Tanner Springs, Arizona
- Tanner's Settlement, Nova Scotia
- Tanner's squeaker
- Tanner's Sumach
- Tanner State Park
- Tanner, Steve
- Tanners' Tower
- Tannersville Railroad Station
- Tannersville Railroad Station (New York)
- Tannersville station (disambiguation)
- Tanner - The Rebellion
- Tanner Thompson
- Tanner Thorson
- Tanner Township
- Tanner Vallejo
- Tanner vs. Gibbler
- Tanner Whitehouse method
- Tanner, Xavier
- Tannery Brook
- Tannery's theorem
- Tannet
- Tannghrishon
- Tannhauser ballad
- Tannhauser folk ballad
- Tannheim valley