- Sample Preparation Solid state synthesis Single crystal growth Chemical routes Thin films
- Applications Photovoltaics
- References
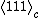 ) with a magnitude of 90–95 μC/cm2.[5][6]
) with a magnitude of 90–95 μC/cm2.[5][6]Sample Preparation
Bismuth ferrite is not a naturally occurring mineral and several synthesis routes to obtain the compound have been developed.
Solid state synthesis
In the solid state reaction method[7] bismuth oxide (Bi2O3) and iron oxide (Fe2O3) in a 1:1 mole ratio are mixed with a mortar, or by ball milling and then fired at elevated temperatures. The volatility of bismuth and the relatively stable competing ternary phases Bi25FeO39 (sillenite) and Bi2Fe4O9 (mullite) makes the solid state synthesis of phase pure and stoichiometric bismuth ferrite challenging. Typically a firing temperature of 800 to 880 Celsius is used for 5 to 60 minutes with rapid subsequent cooling. Excess Bi2O3 has also been used a measure to compensate for bismuth volatility and to avoid formation of the Bi2Fe4O9 phase.
Single crystal growth
Bismuth ferrite melts incongruently, but it can be grown from a bismuth oxide rich flux (e.g. a 4:1:1 mixture of Bi2O3, Fe2O3 and B2O3 at approximately 750-800 Celsius).[8] High quality single crystals have been important for studying the ferroelectric, antiferromagnetic and magnetoelectric properties of bismuth ferrite.
Chemical routes
Wet chemical synthesis routes based on sol-gel chemistry, modified Pechini routes[9] or hydrothermal[10] synthesis have been used to prepare phase pure BiFeO3. The advantage of the chemical routes is the compositional homogeneity of the precursors and the reduced loss of bismuth due to the much lower temperatures needed. In sol-gel routes, an amorphous precursor is calcined at 300-600 Celsius to remove organic residuals and to promote crystallization of the bismuth ferrite perovskite phase, while the disadvantage is that the resulting powder must be sintered at high temperature to make a dense polycrystal.
Solution combustion reaction is a low-cost method used to synthesize porous BiFeO3. In this method, a reducing agent (such glycine, citric acid, urea, etc) and an oxidizing agent (nitrate ions, nitric acid, etc) are used to generate the reduction-oxidation (RedOx) reaction. The appearance of the flame, and consequently the temperature of the mixture, depends on the oxidizing/reducing agents ratio used.[11] Annealing up to 600 °C is sometimes needed to decompose the bismuth oxo-nitrates generated as intermediates. Since the content of Fe cations in this semiconductor material, Mӧssbauer spectroscopy is a proper technique to detect the presence of a paramagnetic component in the phase.
Thin films
The electric and magnetic properties of high quality epitaxial thin films of bismuth ferrite reported in 2003[12] revived the scientific interest for bismuth ferrite. Epitaxial thin films have the great advantage that they can be integrated in electronic circuitry. Epitaxial strain induced by singly crystalline substrates with different lattice parameters than bismuth ferrite can be used to modify the crystal structure to monoclinic or tetragonal symmetry and change the ferroelectric, piezoelectric or magnetic properties.[13] Pulsed laser deposition (PLD) is a very common route to epitaxial BiFeO3 films, and SrTiO3 substrates with SrRuO3 electrodes are typically used. Sputtering, metal organic chemical vapor deposition (MOCVD), atomic layer deposition (ALD), and chemical solution deposition are other methods to prepare epitaxial bismuth ferrite thin films. Apart from its magnetic and electric properties bismuth ferrite also possesses photovoltaic properties which is known as ferroelectric photovoltaic (FPV) effect.
Applications
Being a room temperature multiferroic material and due to its ferroelectric photovoltaic (FPV) effect, bismuth ferrite has several applications in the field of magnetism, spintronics, photovoltaics, etc.
Photovoltaics
In the FPV effect, a photocurrent is generated in a ferroelectric material under illumination and its direction is dependent upon the ferroelectric polarization of that material. The FPV effect has a promising potential as an alternative to conventional photovoltaic devices. But the main hindrance is that a very small photocurrent is generated in ferroelectric materials like LiNbO3[14], which is due to its large bandgap and low conductivity. In this direction bismuth ferrite has shown a great potential since a large photocurrent effect and above bandgap voltage[15] is observed in this material under illumination. Most of the works using bismuth ferrite as a photovoltaic material has been reported on its thin film form but in a few reports researchers have formed a bilayer structure with other materials like polymers, graphene and other semiconductors. In a report p-i-n heterojunction has been formed with bismuth ferrite nanoparticles along with two oxide based carrier transporting layers[16]. In spite of such efforts the power conversion efficiency obtained from bismuth ferrite is still very low.
References
2. ^D. Varshney, A. Kumar, K. Verma, Effect of A site and B site doping on structural, thermal, and dielectric properties of BiFeO3 ceramics, https://doi.org/10.1016/j.jallcom.2011.05.106
3. ^{{cite journal|last1=Kiselev|first1=S. V.|last2=Ozerov|first2=R. P.|last3=Zhdanov|first3=G. S.|title=Detection of magnetic order in ferroelectric BiFeO3 by neutron diffraction|journal=Soviet Physics - Doklady|date=February 1963|volume=7|issue=8|pages=742–744|bibcode=1963SPhD....7..742K}}
4. ^{{cite journal|last1=Spaldin|first1=Nicola A.|author1-link=Nicola Spaldin|last2=Cheong|first2=Sang-Wook|last3=Ramesh|first3=Ramamoorthy|title=Multiferroics: Past, present, and future|journal=Physics Today|date=1 January 2010|volume=63|issue=10|page=38|doi=10.1063/1.3502547|url=http://www.mendeley.com/research/multiferroics-past-present-future-feature/|accessdate=15 February 2012|bibcode = 2010PhT....63j..38S }}
5. ^{{cite journal|last1=Chu|first1=Ying-Hao|first2=Lane W.|last2=Martin|first3=Mikel B.|last3=Holcomb|first4=Ramamoorthy|last4=Ramesh|title=Controlling magnetism with multiferroics|journal=Materials Today|year=2007|volume=10|issue=10|pages=16–23|url=http://www.if.ufrgs.br/~magusmao/FIP10604/RefsSemin/MFreview2.pdf|doi=10.1016/s1369-7021(07)70241-9}}{{dead link|date=July 2017 |bot=InternetArchiveBot |fix-attempted=yes }}
6. ^{{cite journal |last1=Seidel|first1=J. |last2=Martin|first2=L. W.|last3=He|first3=Q. |last4=Zhan|first4=Q. |last5=Chu|first5=Y.-H. |last6=Rother|first6=A. |last7=Hawkridge|first7=M. E. |last8=Maksymovych|first8=P. |last9=Yu|first9=P. |last10=Gajek|first10=M. |last11=Balke|first11=N. |last12=Kalinin|first12=S. V. |last13=Gemming|first13=S. |last14=Wang|first14=F. |last15=Catalan|first15=G. |last16=Scott|first16=J. F. |last17=Spaldin|author17-link=Nicola Spaldin|first17=N. A. |last18=Orenstein|first18=J. |last19=Ramesh|first19=R. |title=Conduction at domain walls in oxide multiferroics|journal=Nature Materials|year=2009|volume=8|issue=3|pages=229–234|doi=10.1038/NMAT2373|bibcode=2009NatMa...8..229S |pmid=19169247}}
7. ^{{cite journal | title = Effect of Pr substitution on structural and electrical properties of BiFeO3 ceramics | doi = 10.1016/j.matchemphys.2013.09.045 | first1 = Poorva |last1 = Sharma | first2 = Dinesh |last2 = Varshney | first3 = S. | last3 = Satapathy | first4 = P.K. | last4 = Gupta | journal=Materials Chemistry and Physics | volume = 143 | issue = 2 | date = 15 January 2014 | pages = 629–636 }}
8. ^1 {{cite journal|last1=Kubel |first1=Frank | first2=Hans |last2 = Schmid|title=Structure of a Ferroelectric and Ferroelastic Monodomain Crystal of the Perovskite BiFeO3|journal=Acta Crystallographica | year=1990|volume=B46 |issue=6 | pages = 698–702 | url = http://archive-ouverte.unige.ch/unige:31426 | doi = 10.1107/S0108768190006887 }}
9. ^{{cite journal | last1 = Ghosh | first1 = Sushmita| last2 = Dasgupta | first2 = Subrata | last3 = Sen | first3 = Amarnath | last4 = Sekhar Maiti | first4 = Himadri | title = Low-Temperature Synthesis of Nanosized Bismuth Ferrite by Soft Chemical Route | journal = Journal of the American Ceramic Society | date = 1 May 2005 | origyear = 14 April 2005 | volume = 88 | issue = 5 | pages = 1349–1352 | doi = 10.1111/j.1551-2916.2005.00306.x }}
10. ^{{cite journal | last1 = Han | first1 = J.-T. | last2 = Huang | first2 = Y.-H. | last3 = Wu | first3 = X.-J. | last4 = Wu | first4 = C.-L. | last5 = Wei | first5 = W. | last6 = Peng | first6 = B. | last7 = Huang | first7 = W. | last8 = Goodenough | first8 = J. B. | title = Tunable Synthesis of Bismuth Ferrites with Various Morphologies | journal = Advanced Materials | date = 18 August 2006 | origyear = 18 July 2006 | volume = 18 | issue = 16 | pages = 2145–2148 | doi = 10.1002/adma.200600072 }}
11. ^{{cite journal |last1=Ortiz-Quiñonez |first1=José-Luis |last2=Pal |first2=Umapada |last3=Villanueva |first3=Martin Salazar |title=Effects of Oxidizing/Reducing Agent Ratio on Phase Purity, Crystallinity, and Magnetic Behavior of Solution-Combustion-Grown BiFeO Submicroparticles |journal=Inorganic Chemistry |date=10 May 2018 |volume=57 |issue=10 |pages=6152–6160 |doi=10.1021/acs.inorgchem.8b00755}}
12. ^1 {{cite journal | last1 = Wang | first1 = J. | first2 = B. | last2 = Neaton | first3 = H. | last3 = Zheng | first4 = V. | last4 = Nagarajan | first5 = S. B. | last5 = Ogale | first6 = B. | last6 = Liu | first7 = D. | last7 = Viehland | first8 = V. | last8 = Vaithyanathan | first9 = D. G. | last9 = Schlom | first10 = U. V. | last10 = Waghmare | first11 = N. A. | last11 = Spaldin|author11-link=Nicola Spaldin | first12 = K. M. | last12 = Rabe | first13 = M. | last13 = Wuttig | first14 = R. | last14 = Ramesh | title = Epitaxial BiFeO3 Multiferroic Thin Film Heterostructures | journal = Science | date = 14 March 2003 | volume = 299 | issue = 5613 | pages = 1719–1722 | doi = 10.1126/science.1080615 | pmid = 12637741 |bibcode = 2003Sci...299.1719W }}
13. ^{{cite journal | last1 = Zeches | first1 = R. J.| last2 = Rossell | first2 = M. D. | last3 = Zhang | first3 = J. X. | last4 = Hatt | first4 = A. J. | last5 = He | first5 = Q. | last6 = Yang | first6 = C.-H. | last7 = Kumar | first7 = A. | last8 = Wang | first8 = C. H. | last9 = Melville | first9 = A. | last10 = Adamo | first10 = C. | last11 = Sheng | first11 = G. | last12 = Chu | first12 = Y.-H. | last13 = Ihlefeld | first13 = J. F. | last14 = Erni | first14 = R. | last15 = Ederer | first15 = C. | last16 = Gopalan | first16 = V. | last17 = Chen | first17 = L. Q. | last18 = Schlom | first18 = D. G. | last19 = Spaldin | first19 = N. A.|author19-link=Nicola Spaldin | last20 = Martin | first20 = L. W. | last21 = Ramesh | first21 = R. | title = A Strain-Driven Morphotropic Phase Boundary in BiFeO3 | journal = Science | date = 12 November 2009 | volume = 326 | issue = 5955 | pages = 977–980 | doi = 10.1126/science.1177046 | pmid = 19965507 |bibcode = 2009Sci...326..977Z }}
14. ^M. Glass, Von der Linde and T. J. Negran, High‐voltage bulk photovoltaic effect and the photorefractive process in LiNbO3,Appl. Phys. Lett.doi:10.1063/1.1655453
15. ^{{cite journal|last1=Yang|first1=S.Y.|last2=Seidel|first2=J.|last3=Byrnes|first3=S.J.|last4=Shafer|first4=P.|last5=Yang|first5=C.H.|last6=Rossell|first6=M.D.|last7=Yu|first7=P.|last8=Chu|first8=Y.H.|last9=Scott|first9=J.F.|last10=Ager|first10=J.W.|last11=Martin|first11=L.W.|last12=Ramesh|first12=R.|title=Above-Bandgap Voltages from Ferroelectric Photovoltaic Devices.|journal=Nature Nanotechnology|volume=5|issue=2|year=2010|pages=143–147|doi=10.1038/nnano.2009.451|pmid=20062051|bibcode = 2010NatNa...5..143Y }}
16. ^{{cite journal|last1=Chatterjee|first1=S.|last2=Bera|first2=A.|last3=Pal|first3=A.J.|title=p–i–n Heterojunctions with BiFeO3 Perovskite Nanoparticles and p- and n-Type Oxides: Photovoltaic Properties|journal=ACS Applied Materials & Interfaces|volume=6|issue=22|year=2014|pages=20479–20486|doi=10.1021/am506066m|pmid=25350523}}
https://doi.org/10.1016/j.jallcom.2011.05.106
- 1990-91 St. John's Redmen basketball team
- 1990-91 Stoke City F.C. season
- 1990-91 Tottenham Hotspur F.C. season
- 1990-91 Tranmere Rovers F.C. season
- 1990-91 U.C. Sampdoria season
- 1990-91 UE Lleida season
- 1990/91 United States network television schedule
- 1990-91 West Ham United F.C. season
- 1990-91 Yugoslav Ice Hockey League season
- 1990-98 Indonesian military operations in Aceh
- 1990-99 world oil market chronology
- 1990 ABC Championship for Women
- 1990 ABN AMRO World Tennis Tournament
- 1990 ABN World Tennis Tournament
- 1990 ABN World Tennis Tournament - Doubles
- 1990 ABN World Tennis Tournament - Singles
- 1990 ABN World Tennis Tournament – Doubles
- 1990 ABN World Tennis Tournament – Singles
- 1990 AFC U-17 Championship
- 1990 AFL Draft
- 1990 Africa Cup of Nations Final
- 1990 Africa Cup of Nations qualification
- 1990 African Cup of Nations Final
- 1990 African Cup of Nations qualification
- 1990 Air Canada Cup