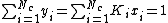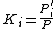- Calculating the bubble point
- References
- See also
In thermodynamics, the bubble point is the temperature (at a given pressure) where the first bubble of vapor is formed when heating a liquid consisting of two or more components.[1][2] Given that vapor will probably have a different composition than the liquid, the bubble point (along with the dew point) at different compositions are useful data when designing distillation systems.[3]
For a single component the bubble point and the dew point are the same and are referred to as the boiling point.
Calculating the bubble point
At the bubble point, the following relationship holds:
where
.
K is the distribution coefficient or K factor, defined as the ratio of mole fraction in the vapor phase 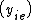 to the mole fraction in the liquid phase
to the mole fraction in the liquid phase  at equilibrium.
at equilibrium.
When Raoult's law and Dalton's law hold for the mixture, the K factor is defined as the ratio of the vapor pressure to the total pressure of the system:[1]
Given either of  or
or  and either the temperature or pressure of a two-component system, calculations can be performed to determine the unknown information.[4]
and either the temperature or pressure of a two-component system, calculations can be performed to determine the unknown information.[4]
References
2. ^{{Citation | last1 = Smith| first1 = J. M. | last2 = Van Ness| first2 = H. C. | last3 = Abbott| first3 = M. M. | title = Introduction to Chemical Engineering Thermodynamics | place = New York | publisher = McGraw-Hill | year = 2005 | location = | pages = 342 | volume = | edition = seventh | url = | doi = | id = | isbn = 0-07-310445-0}}
3. ^{{cite book|editor=Perry, R.H.|editor2=Green, D.W.|title=Perry's Chemical Engineers' Handbook|edition=7th|publisher=McGraw-hill|date=1997|isbn=0-07-049841-5}}
4. ^{{Citation | last1 = Smith| first1 = J. M. | last2 = Van Ness| first2 = H. C. | last3 = Abbott| first3 = M. M. | title = Introduction to Chemical Engineering Thermodynamics | place = New York | publisher = McGraw-Hill | year = 2005 | location = | pages = 351 | volume = | edition = seventh | url = | doi = | id = | isbn = 0-07-310445-0}}
See also
- Phase diagram
- Azeotrope
- Dew point
- University of Kentucky College of Communication & Information
- University of Kentucky College of Dentistry
- University of Kentucky College of Design
- University of Kentucky College of Engineering
- University of Kentucky Oral History
- University of Kentucky Salvation Army Clinic
- University of Kentucky Solar Car Team
- University of Kentucky Student Life
- University of Kentucky Wesley Foundation
- University of Kerla
- University of Khost
- University of Kielce
- University of kings college
- University of Kingston
- University of Kingston-upon-Hull
- University of Kirkuk
- University of Kitakyūshū
- University of Klaipeda
- University of Klaipėda
- University of Kobe
- University of Kochi
- University of Koeln
- University of Koln
- University of Korea
- University of Korça