心音和心音图
用听诊器在健康人胸壁心前区听诊时,可在每一心动周期中听到两个声音,分别称为第一心音和第二心音。第一心音是由房室瓣关闭及心室肌收缩使血液在心脏内振动而产生的声音。与第二心音比较,音调较低、时间较长(约0.12秒)、声音较响,在心尖部听诊最清楚。由于房室瓣在心室开始收缩后几乎是立即关闭,因而第一心音可做为心室开始收缩的标志,其响度和性质常可反映心室收缩的强弱和房室瓣的机能状态。第二心音主要是半月瓣关闭时血液在大动脉根部及心室内振动所引起的。与第一心音比较,音调较高、时间较短(约0.08)秒,在心底部听诊较清楚。由于半月瓣几乎在心室舒张后立即关闭,因而第二心音可作为心室开始舒张的标志,其响度和性质的变化常可反映动脉压的高低及半月瓣的机能状态。由于第一心音标志心室收缩开始,第二心音标志心室舒张开始,因此从第一心音开始到第二心音开始之间的时间相当于心缩期,而从第二心音开始到下一个第一心音开始之间的时间相当于心舒期。在部分正常人中,有时在第二心间之后,还可听到一个短而弱的声音,酷似第二心音的回音,称为第三心音,与心室舒张早期血液急速充盈有关。用一台心音图仪,可将听到的心音转变成记录出的图形,称为心音图。正常的心音图除能记录到第一、第二和第三心音外,还可记录到第四心音(在第一心音之前,是由心房收缩产生的)。心音图的客观性强,对心音中某些频率成分比听诊灵敏,特别是在心率过快时与心电图(见图1.5-4)配合,能判断多种心音。但应注意,并不是任何心音频率成分都是心音图敏感,因为有一些频率成分不及人耳敏感,故听到的心音与描记的心音成分有时会有差异,所以听心音和记录心音图可互为补充。
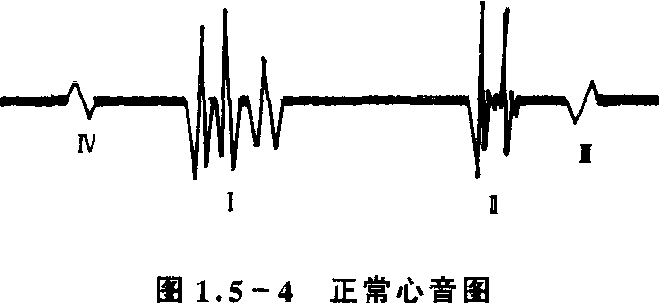
图1.5-4 正常心音图
- Froding,Gustaf
- frog
- frog-bit
- frogfish
- froghopper
- frogmouth
- Frogs
- Froissart,Jean
- Fromm,Erich
- Fronde
- Frondizi,Arturo
- front
- Front de Liberation Nationale
- Frontenac,Louis de Buade,Comte de Palluau et de
- fronton games
- frost
- frostbite
- Frost,RobertLee
- froth notation
- frottola
- Froude,James Anthony
- frozen foods
- Fructidor
- fructose
- fruit