灭线
各种角度的直线或平面伸展至无限远,出现许多消灭点,最后都消灭在这条直线上,此线称为灭线。可分为水平,斜面,竖面等灭线(见图2
❾)。
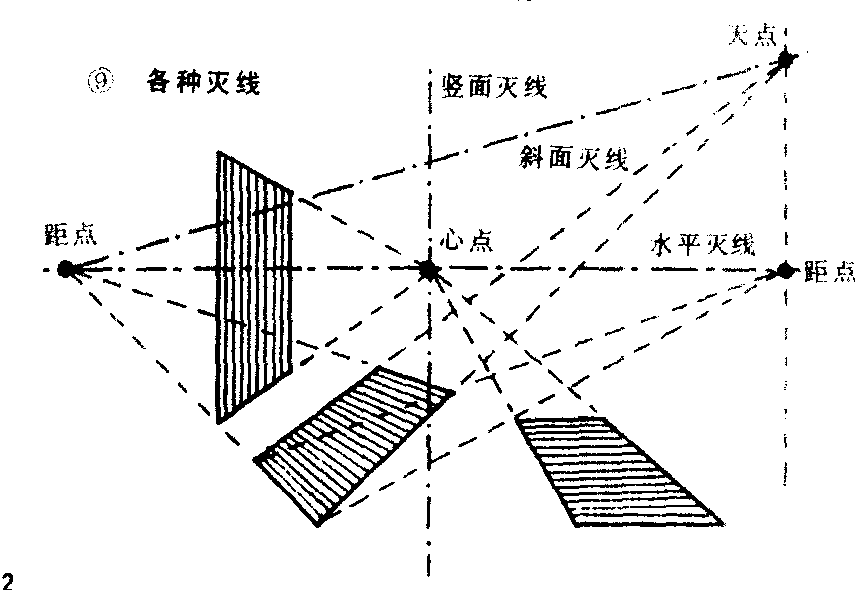
灭线Mie xian
各种角度的直线或平面至无限远处,最后消灭在共同的一条假设的直线上,此直线称为灭线。可分为水平灭线(视平线)、斜面灭线,竖面灭线等。灭线与消灭线有根本的不同,不能混淆图3
❺。
- Garrick,David
- Garrison,William Lloyd
- garter snake
- Garter,Order of the
- Garvey,Marcus
- Gary
- Gary,Elbert Henry
- gas
- Gasca,Pedro de la
- Gascoigne,George
- gas constant
- Gascony
- Gaskell,ElizabethCleghorn
- gas laws
- gas mask
- gas meter
- gasohol
- gas oil
- gasoline
- Gaspee
- Gaspe Peninsula
- Gasperi,Alcide de
- gas plant
- Gassendi,Pierre
- Gasser,Herbert Spencer