环己烷huanjiwan
一种环烷烃,又名六氢化苯,分子式C6H12。无色易挥发易燃的液体,有气油气味; 熔点为6.5℃,沸点为80.7℃,相对密度为0.7785; 不溶于水,与乙醇、乙醚等许多有机溶剂
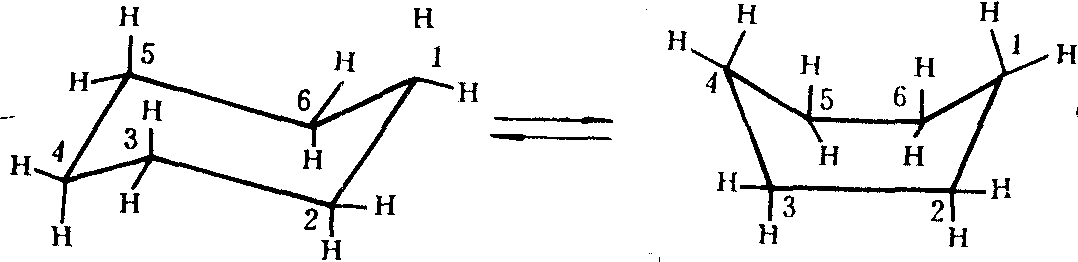
椅式 船式
环己烷的椅式和船式构象
环己烷的化学性质与烷烃相似,能发生卤代反应; 在硝酸作用下氧化为己二酸; 在钴催化剂作用下被空气氧化,生成环己醇和环己酮,后者是合成尼龙6和尼龙66的原料; 在铂或钯催化剂作用下脱氢生成苯。
在工业上环己烷主要由苯催化加氢制得,也可以由石油或裂化汽油中蒸馏提取。其主要用于制取尼龙66和尼龙6的单体——己二酸、己二胺和己内酰胺; 它可以溶解许多有机物,并且毒性比苯小,是一种良好的溶剂,常用做提取香精油以及树脂、涂料、清漆和制造聚乙烯等的溶剂。