- See also
- References
In condensed matter physics and inorganic chemistry the cation-anion radius ratio (also: radius ratio rule [1]) is the ratio of the ionic radius of the cation to the ionic radius of the anion in a cation-anion compound. This is simply given by 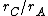 .
.
According to Pauling's rules for crystal structures, the allowed size of the cation for a given structure is determined by the critical radius ratio.[2] If the cation is too small, then it will attract the anions into each other and they will collide hence the compound will be unstable due to anion-anion repulsion; this occurs when the radius ratio drops below 0.155.
At the stability limit the cation is touching all the anions and the anions are just touching at their edges (radius ratio = 0.155). For radius ratios greater than 0.155, the compound may be stable.
The table below gives the relation between radius ratio and coordination number, which may be obtained from a simple geometrical proof.[3]
| Radius Ratio | Coordination number | Type of void | Example |
|---|---|---|---|
| < 0.155 | 2 | Linear | |
| 0.155 - 0.225 | 3 | Triangular Planar | B2O3 |
| 0.225 - 0.414 | 4 | Tetrahedral | ZnS, CuCl |
| 0.414 - 0.732 | 6 | Octahedral | NaCl, MgO |
| 0.732 - 1.000 | 8 | Cubic | CsCl, NH4Br |
The radius ratio rule was first proposed by Gustav F. Hüttig in 1920. [4] [5] In 1926 Victor Goldschmidt [4] extended the use to ionic lattices. [6] [7] [8]
See also
Face-centered cubicReferences
2. ^Linus Pauling (1960) {{Google books|L-1K9HmKmUUC&PA544|Nature of the Chemical Bond|page=544}}
3. ^Toofan J. (1994) Journal of Chemical Education 71(2): 147 {{DOI|10.1021/ed071p147}} (and Erratum 71(9): 749 {{DOI|10.1021/ed071p749}}) A Simple Expression between Critical Radius Ratio and Coordination Numbers
4. ^1 The Origin of the Ionic-Radius Ratio Rules William B. Jensen, Journal of Chemical Education 2010 87 (6), 587-588 {{DOI|10.1021/ed100258f}}
5. ^Hüttig, G. F. (1920), Notiz zur Geometrie der Koordinationszahl. Z. Anorg. Allg. Chem., 114: 24–26. {{doi|10.1002/zaac.19201140103}}
6. ^V. Goldschmidt, T. Barth, G. Lunde, W. Zachariasen, Geochemische Verteilungsgesetze der Elemente. VII. Die Gesetze der Krystallochemie, Dybwad: Oslo, 1926, pp. 112-117.
7. ^V. Goldschmidt, Geochemische Verteilungsgesetze der Elemente. VIII. Untersuchungen über Bau und Eigenschaften von Krystallen, Dybwad: Oslo, 1927, pp. 14-17
8. ^V. Goldschmidt, “Crystal Structure and Chemical Constitution,” Trans. Faraday Soc. 1929, 25, 253-283. {{doi|10.1039/TF9292500253}}
- Vasastaden
- Vasastaden, Gothenburg
- Vasastaden, Göteborg
- Vasastaden, Stockholm
- Vasastan (disambiguation)
- Vasastan, Goeteborg
- Vasastan, Goteborg
- Vasa syndrome
- Vasa Township, Minnesota
- Vasau River
- Vasava
- Vasavad
- Vasavadatta
- Vasa vasora
- Vasavere
- Vasavi Bhil
- Vasavi Kanyaka Parameswari
- Vasavilan
- Vasavi language
- Vasa Zivkovic
- Vasa övningsskola
- Vasa Živković
- Vasbert Conniel Drakes
- Vasby Centrum
- Vasby IK