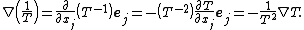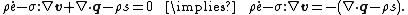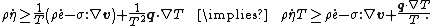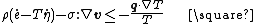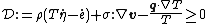- Clausius–Duhem inequality in terms of the specific entropy
- Clausius–Duhem inequality in terms of specific internal energy
- Dissipation
- See also
- References
- External links
The Clausius–Duhem inequality[1][2] is a way of expressing the second law of thermodynamics that is used in continuum mechanics. This inequality is particularly useful in determining whether the constitutive relation of a material is thermodynamically allowable.[3]
This inequality is a statement concerning the irreversibility of natural processes, especially when energy dissipation is involved. It was named after the German physicist Rudolf Clausius and French physicist Pierre Duhem.
Clausius–Duhem inequality in terms of the specific entropy
The Clausius–Duhem inequality can be expressed in integral form as
In this equation  is the time,
is the time, 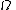 represents a body and the integration is over the volume of the body,
represents a body and the integration is over the volume of the body, 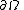 represents the surface of the body,
represents the surface of the body,  is the mass density of the body,
is the mass density of the body, 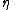 is the specific entropy (entropy per unit mass),
is the specific entropy (entropy per unit mass), 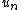 is the normal velocity of
is the normal velocity of 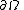 ,
,  is the velocity of particles inside
is the velocity of particles inside 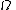 ,
,  is the unit normal to the surface,
is the unit normal to the surface,  is the heat flux vector,
is the heat flux vector,  is an energy source per unit mass, and
is an energy source per unit mass, and  is the absolute temperature. All the variables are functions of a material point at
is the absolute temperature. All the variables are functions of a material point at  at time
at time  .
.
In differential form the Clausius–Duhem inequality can be written as
where 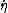 is the time derivative of
is the time derivative of 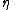 and
and 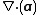 is the divergence of the vector
is the divergence of the vector  .
.
| Proof |
|---|
Assume that  is an arbitrary fixed control volume. Then is an arbitrary fixed control volume. Then
Using the divergence theorem, we get Since Expanding out or, or, Now, the material time derivatives of Therefore, From the conservation of mass |
Clausius–Duhem inequality in terms of specific internal energy
The inequality can be expressed in terms of the internal energy as
where  is the time derivative of the specific internal energy
is the time derivative of the specific internal energy  (the internal energy per unit mass),
(the internal energy per unit mass),  is the Cauchy stress, and
is the Cauchy stress, and 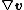 is the gradient of the velocity. This inequality incorporates the balance of energy and the balance of linear and angular momentum into the expression for the Clausius–Duhem inequality.
is the gradient of the velocity. This inequality incorporates the balance of energy and the balance of linear and angular momentum into the expression for the Clausius–Duhem inequality.
| Proof |
|---|
Using the identity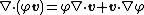 in the Clausius–Duhem inequality, we get Now, using index notation with respect to a Cartesian coordinate system Hence, From the balance of energy Therefore, Rearranging, |
Dissipation
The quantity
is called the dissipation which is defined as the rate of internal entropy production per unit volume times the absolute temperature. Hence the Clausius–Duhem inequality is also called the dissipation inequality. In a real material, the dissipation is always greater than zero.
See also
- Entropy
- Second law of thermodynamics
References
2. ^{{Citation |last=Truesdell |first=Clifford |lastauthoramp=yes |first2=Richard |last2=Toupin |year=1960 |chapter=The Classical Field Theories of Mechanics |title=Handbuch der Physik |volume=III |location=Berlin |publisher=Springer |isbn= }}.
3. ^{{Citation |last=Frémond |first=M. |year=2006 |chapter=The Clausius–Duhem Inequality, an Interesting and Productive Inequality |title=Nonsmooth Mechanics and Analysis |series=Advances in mechanics and mathematics |volume=12 |pages=107–118 |location=New York |publisher=Springer |doi=10.1007/0-387-29195-4_10 |isbn=0-387-29196-2 }}.
External links
- [https://web.archive.org/web/20080513152511/http://www.mechanics.rutgers.edu/TruesdellMemories.pdf Memories of Clifford Truesdell] by Bernard D. Coleman, Journal of Elasticity, 2003.
- Thoughts on Thermomechanics by Walter Noll, 2008.
- Westwood Primary School
- Westwood School District
- Westwood School District (Michigan)
- Westwood Secondary School - Malton
- Westwoodside
- Westwood, Southfleet
- Westwood (subdivision), Houston
- Westwood's White-lady
- Westwood Town Center Historic District
- Westwood (UCLA)
- Westwood, Utah
- West Wood, Utah
- Westwood, William
- Westwood, Wiltshire
- West Woodyates
- Westworld (TV series)
- West Worlington
- West WR1000
- West Wyalong (Drysdale)
- West Wyalong, New South Wales
- West Wyalong railway station
- West Wycombe Caves
- West Wycombe House Park
- West Wykeham
- West Wykeham, Ludford, Lincolnshire
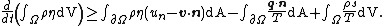
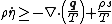
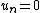 and the derivative can be taken inside the integral to give
and the derivative can be taken inside the integral to give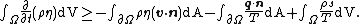
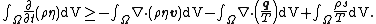
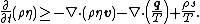
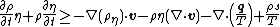
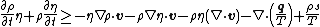
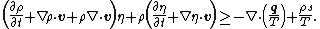
 and
and  are given by
are given by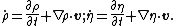
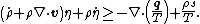
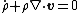 . Hence,
. Hence,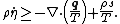
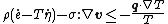
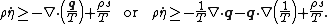
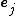 ,
,