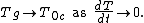- Introduction
- Transition temperature Tg Polymers Silicates and other covalent network glasses
- Kauzmann's paradox Alternative resolutions
- In specific materials Silica, SiO2 Polymers
- Mechanics of vitrification Electronic structure
- References
- External links
The glass–liquid transition, or glass transition, is the gradual and reversible transition in amorphous materials (or in amorphous regions within semicrystalline materials), from a hard and relatively brittle "glassy" state into a viscous or rubbery state as the temperature is increased.[1] An amorphous solid that exhibits a glass transition is called a glass. The reverse transition, achieved by supercooling a viscous liquid into the glass state, is called vitrification.
The glass-transition temperature Tg of a material characterizes the range of temperatures over which this glass transition occurs. It is always lower than the melting temperature, Tm, of the crystalline state of the material, if one exists.
Hard plastics like polystyrene and poly(methyl methacrylate) are used well below their glass transition temperatures, i.e., when they are in their glassy state. Their Tg values are well above room temperature, both at around {{Convert|100|C}}. Rubber elastomers like polyisoprene and polyisobutylene are used above their Tg, that is, in the rubbery state, where they are soft and flexible.[2]
Despite the change in the physical properties of a material through its glass transition, the transition is not considered a phase transition; rather it is a phenomenon extending over a range of temperature and defined by one of several conventions.[3][4] Such conventions include a constant cooling rate ({{Convert|20|K/min|F/min}})[1] and a viscosity threshold of 1012 Pa·s, among others. Upon cooling or heating through this glass-transition range, the material also exhibits a smooth step in the thermal-expansion coefficient and in the specific heat, with the location of these effects again being dependent on the history of the material.[6] The question of whether some phase transition underlies the glass transition is a matter of continuing research.[3][4][5]
{{Quote box| title = IUPAC definition
| quote = glass transition (in polymer science): Process in which a polymer melt changes on cooling to a polymer glass or a polymer glass changes on heating to a polymer melt.[6]
Note 1: Phenomena occurring at the glass transition of polymers are still subject to ongoing scientific investigation and debate. The glass transition presents features of a second-order
transition since thermal studies often indicate that the molar Gibbs energies, molar
enthalpies, and the molar volumes of the two phases, i.e., the melt and the glass, are
equal, while the heat capacity and the expansivity are discontinuous. However, the glass
transition is generally not regarded as a thermodynamic transition in view of the inherent difficulty in reaching equilibrium in a polymer glass or in a polymer melt at temperatures close to the glass-transition temperature.
Note 2: In the case of polymers, conformational changes of segments, typically consisting of
10–20 main-chain atoms, become infinitely slow below the glass transition temperature.
Note 3: In a partially crystalline polymer the glass transition occurs only in the amorphous parts
of the material.
Note 4: The definition is different from that in ref.[7]
Note 5: The commonly used term “glass-rubber transition” for glass transition is not recommended.[8]
| align = right
| width = 30%
}}
Introduction
The glass transition of a liquid to a solid-like state may occur with either cooling or compression.[9] The transition comprises a smooth increase in the viscosity of a material by as much as 17 orders of magnitude within a temperature range of 500 K without any pronounced change in material structure.[10] The consequence of this dramatic increase is a glass exhibiting solid-like mechanical properties on the timescale of practical observation. This transition is in contrast to the freezing or crystallization transition, which is a first-order phase transition in the Ehrenfest classification and involves discontinuities in thermodynamic and dynamic properties such as volume, energy, and viscosity. In many materials that normally undergo a freezing transition, rapid cooling will avoid this phase transition and instead result in a glass transition at some lower temperature. Other materials, such as many polymers, lack a well defined crystalline state and easily form glasses, even upon very slow cooling or compression. The tendency for a material to form a glass while quenched is called glass forming ability. This ability depends on the composition of the material and can be predicted by the rigidity theory.[11]
Below the transition temperature range, the glassy structure does not relax in accordance with the cooling rate used. The expansion coefficient for the glassy state is roughly equivalent to that of the crystalline solid. If slower cooling rates are used, the increased time for structural relaxation (or intermolecular rearrangement) to occur may result in a higher density glass product. Similarly, by annealing (and thus allowing for slow structural relaxation) the glass structure in time approaches an equilibrium density corresponding to the supercooled liquid at this same temperature. Tg is located at the intersection between the cooling curve (volume versus temperature) for the glassy state and the supercooled liquid.[12][13][14][15][16]
The configuration of the glass in this temperature range changes slowly with time towards the equilibrium structure. The principle of the minimization of the Gibbs free energy provides the thermodynamic driving force necessary for the eventual change. It should be noted here that at somewhat higher temperatures than Tg, the structure corresponding to equilibrium at any temperature is achieved quite rapidly. In contrast, at considerably lower temperatures, the configuration of the glass remains sensibly stable over increasingly extended periods of time.
Thus, the liquid-glass transition is not a transition between states of thermodynamic equilibrium. It is widely believed that the true equilibrium state is always crystalline. Glass is believed to exist in a kinetically locked state, and its entropy, density, and so on, depend on the thermal history. Therefore, the glass transition is primarily a dynamic phenomenon. Time and temperature are interchangeable quantities (to some extent) when dealing with glasses, a fact often expressed in the time–temperature superposition principle. On cooling a liquid, internal degrees of freedom successively fall out of equilibrium. However, there is a longstanding debate whether there is an underlying second-order phase transition in the hypothetical limit of infinitely long relaxation times.{{clarify|date=June 2016}}[17][18][19][20]
Transition temperature Tg
{{anchor|Transition temperature}}{{Refimprove section|date=July 2009}}Refer to the figure on the upper right plotting the heat capacity as a function of temperature. In this context, Tg is the temperature corresponding to point A on the curve. The linear sections below and above Tg are colored green. Tg is the temperature at the intersection of the red regression lines.[21]
Different operational definitions of the glass transition temperature Tg are in use, and several of them are endorsed as accepted scientific standards. Nevertheless, all definitions are arbitrary, and all yield different numeric results: at best, values of Tg for a given substance agree within a few kelvins. One definition refers to the viscosity, fixing Tg at a value of 1013 poise (or 1012 Pa·s). As evidenced experimentally, this value is close to the annealing point of many glasses.[22]
In contrast to viscosity, the thermal expansion, heat capacity, shear modulus, and many other properties of inorganic glasses show a relatively sudden change at the glass transition temperature. Any such step or kink can be used to define Tg. To make this definition reproducible, the cooling or heating rate must be specified.
The most frequently used definition of Tg uses the energy release on heating in differential scanning calorimetry (DSC, see figure). Typically, the sample is first cooled with 10 K/min and then heated with that same speed.
Yet another definition of Tg uses the kink in dilatometry (a.k.a. thermal expansion). Here, heating rates of {{Convert|3|-|5|K/min|abbr=on|F/min}} are common. Summarized below are Tg values characteristic of certain classes of materials.
Polymers
| Material | Tg (°C) | Tg (°F) | Commercial name |
|---|---|---|---|
| Tire rubber | -70|C|disp=table}}[23] | ||
| Polyvinylidene fluoride (PVDF) | -35|C|disp=table}}[28] | ||
| Polypropylene (PP atactic) | -20|C|disp=table}}[24] | ||
| Polyvinyl fluoride (PVF) | -20|C|disp=table}}[25] | ||
| Polypropylene (PP isotactic) | 0|C|disp=table}}[24] | ||
| Poly-3-hydroxybutyrate (PHB) | 15|C|disp=table}}[24] | ||
| Poly(vinyl acetate) (PVAc) | 30|C|disp=table}}[24] | ||
| Polychlorotrifluoroethylene (PCTFE) | 45|C|disp=table}}[25] | ||
| Polyamide (PA) | 47|-|60|C|disp=table}} | Nylon-6,x | |
| Polylactic acid (PLA) | 60|-|65|C|disp=table}} | ||
| Polyethylene terephthalate (PET) | 70|C|disp=table}}[24] | ||
| Poly(vinyl chloride) (PVC) | 80|C|disp=table}}[24] | ||
| Poly(vinyl alcohol) (PVA) | 85|C|disp=table}}[24] | ||
| Polystyrene (PS) | 95|C|disp=table}}[24] | ||
| Poly(methyl methacrylate) (PMMA atactic) | 105|C|disp=table}}[24] | Plexiglas, Perspex | |
| Acrylonitrile butadiene styrene (ABS) | 105|C|disp=table}}[26] | ||
| Polytetrafluoroethylene (PTFE) | 115|C|disp=table}}[27] | Teflon | |
| Poly(carbonate) (PC) | 145|C|disp=table}}[24] | Lexan | |
| Polysulfone | 185|C|disp=table}} | ||
| Polynorbornene | 215|C|disp=table}}[24] |
Dry nylon-6 has a glass transition temperature of {{Convert|47|C}}.[28]
Nylon-6,6 in the dry state has a glass transition temperature of about {{Convert|70|C}}.[29][30]
Whereas polyethene has a glass transition range of {{Convert|-130|-|-80|C}}[31]
The above are only mean values, as the glass transition temperature depends on the cooling rate and molecular weight distribution and could be influenced by additives. For a semi-crystalline material, such as polyethene that is 60–80% crystalline at room temperature, the quoted glass transition refers to what happens to the amorphous part of the material upon cooling.
Silicates and other covalent network glasses
| Material | Tg (°C) | Tg (°F) |
|---|---|---|
| Chalcogenide GeSbTe | 150|C|disp=table}}[32] | |
| Chalcogenide AsGeSeTe | 245|C|disp=table}} | |
| ZBLAN fluoride glass | 235|C|disp=table}} | |
| Tellurium dioxide | 280|C|disp=table}} | |
| Fluoroaluminate | 400|C|disp=table}} | |
| Soda-lime glass | 520|-|600|C|disp=table}} | |
| Fused quartz (approximate) | 1200|C|sigfig=2|disp=table}}[33] |
Kauzmann's paradox
As a liquid is supercooled, the difference in entropy between the liquid and solid phase decreases. By extrapolating the heat capacity of the supercooled liquid below its glass transition temperature, it is possible to calculate the temperature at which the difference in entropies becomes zero. This temperature has been named the Kauzmann temperature.
If a liquid could be supercooled below its Kauzmann temperature, and it did indeed display a lower entropy than the crystal phase, the consequences would be paradoxical. This Kauzmann paradox has been the subject of much debate and many publications since it was first put forward by Walter Kauzmann in 1948.[34]
One resolution of the Kauzmann paradox is to say that there must be a phase transition before the entropy of the liquid decreases. In this scenario, the transition temperature is known as the calorimetric ideal glass transition temperature T0c. In this view, the glass transition is not merely a kinetic effect, i.e. merely the result of fast cooling of a melt, but there is an underlying thermodynamic basis for glass formation. The glass transition temperature:
The Gibbs-DiMarzio model specifically predicts that a supercooled liquid's configurational entropy disappears in the limit  , where the liquid's existence regime ends, its microstructure becomes identical to the crystal's, and their property curves intersect in a true second-order phase transition. This has never been experimentally verified due to the difficulty of realizing a slow enough cooling rate while avoiding accidental crystallization.
, where the liquid's existence regime ends, its microstructure becomes identical to the crystal's, and their property curves intersect in a true second-order phase transition. This has never been experimentally verified due to the difficulty of realizing a slow enough cooling rate while avoiding accidental crystallization.
Alternative resolutions
There are at least three other possible resolutions to the Kauzmann paradox. It could be that the heat capacity of the supercooled liquid near the Kauzmann temperature smoothly decreases to a smaller value. It could also be that a first order phase transition to another liquid state occurs before the Kauzmann temperature with the heat capacity of this new state being less than that obtained by extrapolation from higher temperature. Finally, Kauzmann himself resolved the entropy paradox by postulating that all supercooled liquids must crystallize before the Kauzmann temperature is reached.
In specific materials
Silica, SiO2
Silica (the chemical compound SiO2) has a number of distinct crystalline forms in addition to the quartz structure. Nearly all of the crystalline forms involve tetrahedral SiO4 units linked together by shared vertices in different arrangements. Si-O bond lengths vary between the different crystal forms. For example, in α-quartz the bond length is {{convert|161|pm}}, whereas in α-tridymite it ranges from {{convert|154|–|171|pm|abbr=on}}. The Si-O-Si bond angle also varies from 140° in α-tridymite to 144° in α-quartz to 180° in β-tridymite. Any deviations from these standard parameters constitute microstructural differences or variations that represent an approach to an amorphous, vitreous or glassy solid.
The transition temperature Tg in silicates is related to the energy required to break and re-form covalent bonds in an amorphous (or random network) lattice of covalent bonds. The Tg is clearly influenced by the chemistry of the glass. For example, addition of elements such as B, Na, K or Ca to a silica glass, which have a valency less than 4, helps in breaking up the network structure, thus reducing the Tg. Alternatively, P, which has a valency of 5, helps to reinforce an ordered lattice, and thus increases the Tg.[35]
Tg is directly proportional to bond strength, e.g. it depends on quasi-equilibrium thermodynamic parameters of the bonds e.g. on the enthalpy Hd and entropy Sd of configurons – broken bonds: Tg = Hd / [Sd + Rln[(1-fc)/ fc] where R is the gas constant and fc is the percolation threshold. For strong melts such as SiO2 the percolation threshold in the above equation is the universal Scher-Zallen critical density in the 3-D space e.g. fc = 0.15, however for fragile materials the percolation thresholds are material-dependent and fc << 1.[36] The enthalpy Hd and the entropy Sd of configurons – broken bonds can be found from available experimental data on viscosity.[37]Polymers
In polymers the glass transition temperature, Tg, is often expressed as the temperature at which the Gibbs free energy is such that the activation energy for the cooperative movement of 50 or so elements of the polymer is exceeded {{Citation needed|date=September 2010}}. This allows molecular chains to slide past each other when a force is applied. From this definition, we can see that the introduction of relatively stiff chemical groups (such as benzene rings) will interfere with the flowing process and hence increase Tg.[38]
The stiffness of thermoplastics decreases due to this effect (see figure.) When the glass temperature has been reached, the stiffness stays the same for a while, i.e., at or near E2, until the temperature exceeds Tm, and the material melts. This region is called the rubber plateau.
In ironing, a fabric is heated through this transition so that the polymer chains become mobile. The weight of the iron then imposes a preferred orientation. Tg can be significantly decreased by addition of plasticizers into the polymer matrix. Smaller molecules of plasticizer embed themselves between the polymer chains, increasing the spacing and free volume, and allowing them to move past one another even at lower temperatures. The addition of nonreactive side groups to a polymer can also make the chains stand off from one another, reducing Tg. If a plastic with some desirable properties has a Tg that is too high, it can sometimes be combined with another in a copolymer or composite material with a Tg below the temperature of intended use. Note that some plastics are used at high temperatures, e.g., in automobile engines, and others at low temperatures.[24]
In viscoelastic materials, the presence of liquid-like behavior depends on the properties of and so varies with rate of applied load, i.e., how quickly a force is applied. The silicone toy Silly Putty behaves quite differently depending on the time rate of applying a force: pull slowly and it flows, acting as a heavily viscous liquid; hit it with a hammer and it shatters, acting as a glass.
On cooling, rubber undergoes a liquid-glass transition, which has also been called a rubber-glass transition.
Mechanics of vitrification
{{Main|Vitrification}}Molecular motion in condensed matter can be represented by a Fourier series whose physical interpretation consists of a superposition of longitudinal and transverse waves of atomic displacement with varying directions and wavelengths. In monatomic systems, these waves are called density fluctuations. (In polyatomic systems, they may also include compositional fluctuations.)[39]
Thus, thermal motion in liquids can be decomposed into elementary longitudinal vibrations (or acoustic phonons) while transverse vibrations (or shear waves) were originally described only in elastic solids exhibiting the highly ordered crystalline state of matter. In other words, simple liquids cannot support an applied force in the form of a shearing stress, and will yield mechanically via macroscopic plastic deformation (or viscous flow). Furthermore, the fact that a solid deforms locally while retaining its rigidity – while a liquid yields to macroscopic viscous flow in response to the application of an applied shearing force – is accepted by many as the mechanical distinction between the two.[40][41]
The inadequacies of this conclusion, however, were pointed out by Frenkel in his revision of the kinetic theory of solids and the theory of elasticity in liquids. This revision follows directly from the continuous characteristic of the structural transition from the liquid state into the solid one when this transition is not accompanied by crystallization—ergo the supercooled viscous liquid. Thus we see the intimate correlation between transverse acoustic phonons (or shear waves) and the onset of rigidity upon vitrification, as described by Bartenev in his mechanical description of the vitrification process.[42][43]
The velocities of longitudinal acoustic phonons in condensed matter are directly responsible for the thermal conductivity that levels out temperature differentials between compressed and expanded volume elements. Kittel proposed that the behavior of glasses is interpreted in terms of an approximately constant "mean free path" for lattice phonons, and that the value of the mean free path is of the order of magnitude of the scale of disorder in the molecular structure of a liquid or solid. The thermal phonon mean free paths or relaxation lengths of a number of glass formers have been plotted versus the glass transition temperature, indicating a linear relationship between the two. This has suggested a new criterion for glass formation based on the value of the phonon mean free path.[44]
It has often been suggested that heat transport in dielectric solids occurs through elastic vibrations of the lattice, and that this transport is limited by elastic scattering of acoustic phonons by lattice defects (e.g. randomly spaced vacancies).[45]
These predictions were confirmed by experiments on commercial glasses and glass ceramics, where mean free paths were apparently limited by "internal boundary scattering" to length scales of {{convert|10|–|100|µm}}.[46][47]
The relationship between these transverse waves and the mechanism of vitrification has been described by several authors who proposed that the onset of correlations between such phonons results in an orientational ordering or "freezing" of local shear stresses in glass-forming liquids, thus yielding the glass transition.[48]
Electronic structure
The influence of thermal phonons and their interaction with electronic structure is a topic that was appropriately introduced in a discussion of the resistance of liquid metals. Lindemann's theory of melting is referenced, and it is suggested that the drop in conductivity in going from the crystalline to the liquid state is due to the increased scattering of conduction electrons as a result of the increased amplitude of atomic vibration. Such theories of localization have been applied to transport in metallic glasses, where the mean free path of the electrons is very small (on the order of the interatomic spacing).[49][50]
The formation of a non-crystalline form of a gold-silicon alloy by the method of splat quenching from the melt led to further considerations of the influence of electronic structure on glass forming ability, based on the properties of the metallic bond.[51][52][53][54][55]
Other work indicates that the mobility of localized electrons is enhanced by the presence of dynamic phonon modes. One claim against such a model is that if chemical bonds are important, the nearly free electron models should not be applicable. However, if the model includes the buildup of a charge distribution between all pairs of atoms just like a chemical bond (e.g., silicon, when a band is just filled with electrons) then it should apply to solids.[56]
Thus, if the electrical conductivity is low, the mean free path of the electrons is very short. The electrons will only be sensitive to the short-range order in the glass since they do not get a chance to scatter from atoms spaced at large distances. Since the short-range order is similar in glasses and crystals, the electronic energies should be similar in these two states. For alloys with lower resistivity and longer electronic mean free paths, the electrons could begin to sense that there is disorder in the glass, and this would raise their energies and destabilize the glass with respect to crystallization. Thus, the glass formation tendencies of certain alloys may therefore be due in part to the fact that the electron mean free paths are very short, so that only the short-range order is ever important for the energy of the electrons.
It has also been argued that glass formation in metallic systems is related to the "softness" of the interaction potential between unlike atoms. Some authors, emphasizing the strong similarities between the local structure of the glass and the corresponding crystal, suggest that chemical bonding helps to stabilize the amorphous structure.[57][58]
Other authors have suggested that the electronic structure yields its influence on glass formation through the directional properties of bonds. Non-crystallinity is thus favored in elements with a large number of polymorphic forms and a high degree of bonding anisotropy. Crystallization becomes more unlikely as bonding anisotropy is increased from isotropic metallic to anisotropic metallic to covalent bonding, thus suggesting a relationship between the group number in the periodic table and the glass forming ability in elemental solids.[59]
References
2. ^{{Cite web|url=http://pslc.ws/macrog/tg.htm|title=The Glass Transition|publisher=Polymer Science Learning Center}}
3. ^1 {{cite journal|doi=10.1038/35065704|last=Debenedetti|first=P. G.|author2=Stillinger|title=Supercooled liquids and the glass transition|journal=Nature|year=2001|volume=410|issue=6825|pages=259–267|pmid=11258381|bibcode = 2001Natur.410..259D }}
4. ^1 {{cite journal|doi=10.1063/1.1286035|last=Angell|first=C. A.|author2=Ngai, K. L. |author3=McKenna, G. B. |author4=McMillan, P. F. |author5= Martin, S. W. |title=Relaxation in glassforming liquids and amorphous solids|journal=App. Phys. Rev.|year=2000|volume=88|issue=6|pages=3113–3157|bibcode = 2000JAP....88.3113A |url=http://lib.dr.iastate.edu/mse_pubs/70}}
5. ^{{cite journal|doi=10.1134/1.1790021|title=Glass formation in amorphous SiO2 as a percolation phase transition in a system of network defects|year=2004|last1=Ojovan|first1=M. I.|journal=Journal of Experimental and Theoretical Physics Letters|volume=79|issue=12|pages=632–634|bibcode = 2004JETPL..79..632O }}
6. ^{{cite journal |last1=Meille Stefano, V.; Allegra, G.; Geil Phillip, H.; He, J.; Hess, M.; Jin, J.-I.; Kratochvíl, P.; Mormann, W.; Stepto, R. |title=Definitions of terms relating to crystalline polymers (IUPAC Recommendations 2011) |journal=Pure Appl Chem |date=2011 |volume=83 |issue=10 |page=1831 |doi=10.1351/PAC-REC-10-11-13 |url=https://www.degruyter.com/downloadpdf/j/pac.2011.83.issue-10/pac-rec-10-11-13/pac-rec-10-11-13.pdf}}
7. ^{{GoldBookRef |title=glass transition |file=G02640 }}
8. ^{{cite journal |last1=Hess, M.; Allegra, G.; He, J.; Horie, K.; Kim, J.-S.; Meille Stefano, V.; Metanomski, V.; Moad, G.; Stepto Robert, F. T.; Vert, M.; Vohlídal, J. |title=Glossary of terms relating to thermal and thermomechanical properties of polymers (IUPAC Recommendations 2013) |journal=Pure Appl Chem |date=2013|volume=85 |issue=5 |page=1017 |doi=10.1351/PAC-REC-12-03-02 |url=https://www.degruyter.com/downloadpdf/j/pac.2013.85.issue-5/pac-rec-12-03-02/pac-rec-12-03-02.pdf}}
9. ^{{cite book|last=Hansen|first=J.-P.|title=Theory of Simple Liquids| isbn=978-0123705358|url=https://books.google.com/books?id=Uhm87WZBnxEC&printsec=frontcover|year=2007|publisher=Elsevier|pages=250–254|author2=McDonald, I. R.}}
10. ^{{cite book|author1=Adam, J-L |author2=Zhang, X. |title=Chalcogenide Glasses: Preparation, Properties and Applications|url=https://books.google.com/books?id=IuNRAwAAQBAJ&pg=PA94|date=14 February 2014|publisher=Elsevier Science|isbn=978-0-85709-356-1|page=94}}
11. ^{{cite journal|last=Phillips|first=J.C.|title=Topology of covalent non-crystalline solids I: Short-range order in chalcogenide alloys|journal=Journal of Non-Crystalline Solids|year=1979|volume=34|issue=2|page=153|doi=10.1016/0022-3093(79)90033-4|bibcode = 1979JNCS...34..153P }}
12. ^Moynihan, C. et al. (1976) in The Glass Transition and the Nature of the Glassy State, M. Goldstein and R. Simha (Eds.), Ann. N.Y. Acad. Sci., Vol. 279. {{ISBN|0890720533}}.
13. ^{{cite journal|doi=10.1016/0022-3697(88)90002-9|title=Perspective on the glass transition|year=1988|last1=Angell|first1=C. A.|journal=Journal of Physics and Chemistry of Solids|volume=49|issue=8|pages=863–871|bibcode = 1988JPCS...49..863A }}
14. ^{{cite journal|doi=10.1021/jp953538d|title=Supercooled Liquids and Glasses|year=1996|last1=Ediger|first1=M. D.|last2=Angell|first2=C. A.|last3=Nagel|first3=Sidney R.|journal=The Journal of Physical Chemistry|volume=100|issue=31|pages=13200}}
15. ^{{cite journal|doi=10.1126/science.267.5206.1924|title=Formation of Glasses from Liquids and Biopolymers|year=1995|last1=Angell|first1=C. A.|journal=Science|volume=267|issue=5206|pages=1924–35|pmid=17770101|bibcode = 1995Sci...267.1924A }}
16. ^{{cite journal|doi=10.1126/science.267.5206.1935|title=A Topographic View of Supercooled Liquids and Glass Formation|year=1995|last1=Stillinger|first1=F. H.|journal=Science|volume=267|issue=5206|pages=1935–9|pmid=17770102|bibcode = 1995Sci...267.1935S }}
17. ^1 {{Cite book|url=https://books.google.com/books?id=D7Z8ywb3QggC&printsec=frontcover|author=Zarzycki, J. |title=Glasses and the Vitreous State|isbn=978-0521355827 |publisher=Cambridge University Press |year=1991}}
18. ^{{Cite book |vauthors=Nemilov SV |title= Thermodynamic and Kinetic Aspects of the Vitreous State |publisher=CRC Press |year=1994|isbn=978-0849337826}}
19. ^{{Cite book |author=Gibbs, J. H. |editor=MacKenzie, J. D. |title=Modern Aspects of the Vitreous State |publisher=Butterworth |year=1960 |oclc=1690554}}
20. ^{{cite journal|doi=10.1016/j.jnoncrysol.2010.05.012|title=Connectivity and glass transition in disordered oxide systems|year=2010|last1=Ojovan|first1=Michael I|last2=Lee|first2=William (Bill) E|journal=Journal of Non-Crystalline Solids|volume=356|issue=44–49|pages=2534|bibcode = 2010JNCS..356.2534O }}
21. ^Tg measurement of glasses. Glassproperties.com. Retrieved on 2012-06-29.
22. ^{{GoldBookRef |title=glass-transition temperature |file=G02641 }}
23. ^{{Cite journal|url=http://patentscope.wipo.int/search/en/WO2003053721|last1=Galimberti|first1=Maurizio|last2=Caprio|first2=Michela|last3=Fino|first3=Luigi|publicationdate =2003-03-07|date =2001-12-21|title =Tyre comprising a cycloolefin polymer, tread band and elasomeric composition used therein|quote=country-code =EU, patent-number =WO03053721}}
24. ^1 2 3 4 5 6 7 8 9 10 11 {{Cite book|title=PVC Handbook|year=2005|url=https://books.google.com/books?id=YUkJNI9QYsUC&pg=PT1&lpg=PT1|author= Wilkes, C. E.|publisher =Hanser Verlag|isbn=978-1-56990-379-7}}
25. ^1 2 {{cite book | title=THERMOPLASTIC MATERIALS Properties, Manufacturing Methods, and Applications | publisher=CRC Press | author=Ibeh, Christopher C. | year=2011 | pages=491–497 | isbn=978-1-4200-9383-4}}
26. ^[https://web.archive.org/web/20120227150812/https://www.nrri.umn.edu/NLTC/ABS07.pdf ABS]. nrri.umn.edu
27. ^{{cite book | title=The Chemistry of Polymers | publisher=Royal Society of Chemistry | author=Nicholson, John W. | year=2011 | pages=50 | isbn=9781849733915 | url=https://books.google.com/books?id=5XFsT69cX_YC&printsec=frontcover&dq=polymer+chemistry#v=onepage&q=polymer%20chemistry&f=false | edition=4, Revised |accessdate=10 September 2013}}
28. ^nylon-6 information and properties. Polymerprocessing.com (2001-04-15). Retrieved on 2012-06-29.
29. ^{{cite journal | last1 = Jones | first1 = A | year = 2014 | title = Supplementary Materials for Artificial Muscles from Fishing Line and Sewing Thread | url = | journal = Science | volume = 343 | issue = 6173| pages = 868–72 | doi = 10.1126/science.1246906 | pmid = 24558156 |bibcode = 2014Sci...343..868H }}
30. ^[https://web.archive.org/web/20150520110712/http://www.tainstruments.co.jp/application/pdf/Thermal_Library/Applications_Briefs/TA133.PDF Measurement of Moisture Effects on the Mechanical Properties of 66 Nylon]. TA Instruments Thermal Analysis Application Brief TA-133
31. ^PCL | Applications and End Uses | Polythene. Polyesterconverters.com. Retrieved on 2012-06-29.
32. ^EPCOS 2007: Glass Transition and Crystallization in Phase Change Materials . Retrieved on 2012-06-29.
33. ^{{Cite journal| first1 = J. A. | title = High-temperature Brillouin scattering in fused quartz | journal = Journal of Applied Physics| last1 = Bucaro | volume = 45| issue = 12 | pages = 5324–5329 | year = 1974 | doi = 10.1063/1.1663238|bibcode = 1974JAP....45.5324B }}
34. ^{{cite journal|doi=10.1021/cr60135a002|title=The Nature of the Glassy State and the Behavior of Liquids at Low Temperatures|year=1948|last1=Kauzmann|first1=Walter|journal=Chemical Reviews|volume=43|issue=2|pages=219–256}}
35. ^{{Cite journal|author=Ojovan M.I.|title= Configurons: thermodynamic parameters and symmetry changes at glass transition|journal=Entropy|volume=10|issue=3|pages=334–364|year=2008|url=http://www.mdpi.org/entropy/papers/e10030334.pdf|doi=10.3390/e10030334|bibcode = 2008Entrp..10..334O }}
36. ^{{cite journal|author=Ojovan, M.I. |title= Configurons: thermodynamic parameters and symmetry changes at glass transition |journal= Entropy|volume=10|pages= 334–364 |year=2008|url= http://www.mdpi.org/entropy/papers/e10030334.pdf|doi=10.3390/e10030334|issue=3 |bibcode=2008Entrp..10..334O}}
37. ^{{cite journal|doi=10.1088/0953-8984/19/41/415107|pmid=28192319|title=Thermodynamic parameters of bonds in glassy materials from viscosity–temperature relationships|year=2007|last1=Ojovan|first1=Michael I|last2=Travis|first2=Karl P|last3=Hand|first3=Russell J|journal=Journal of Physics: Condensed Matter|volume=19|issue=41|pages=415107|bibcode = 2007JPCM...19O5107O }}
38. ^Cowie, J. M. G. and Arrighi, V., Polymers: Chemistry and Physics of Modern Materials, 3rd Edn. (CRC Press, 2007) {{ISBN|0748740732}}
39. ^Slater, J.C., Introduction to Chemical Physics (3rd Ed., Martindell Press, 2007) {{ISBN|1178626598}}
40. ^{{cite journal|doi=10.1017/S0305004100017138|title=On the stability of crystal lattices. I|year=2008|last1=Born|first1=Max|journal=Mathematical Proceedings of the Cambridge Philosophical Society|volume=36|issue=2|pages=160|bibcode = 1940PCPS...36..160B }}
41. ^{{cite journal| doi=10.1063/1.1750497| title=Thermodynamics of Crystals and Melting| year=1939| last1=Born| first1=Max| journal=The Journal of Chemical Physics| volume=7| issue=8| pages=591–603|bibcode = 1939JChPh...7..591B }}
42. ^{{cite book|author=Frenkel, J.|title=Kinetic Theory of Liquids|publisher=Clarendon Press, Oxford|year=1946}}
43. ^Bartenev, G. M., Structure and Mechanical Properties of Inorganic Glasses (Wolters – Noordhoof, 1970) {{ISBN|9001054501}}
44. ^{{cite journal |author=Reynolds, C. L. Jr. |title=Correlation between the low temperature phonon mean free path and glass transition temperature in amorphous solids |journal=J. Non-Cryst. Solids |volume=30 |issue=3 |page=371 |year=1979 |doi=10.1016/0022-3093(79)90174-1|bibcode = 1979JNCS...30..371R }}
45. ^Rosenburg, H. M. (1963) Low Temperature Solid State Physics. Clarendon Press, Oxford.
46. ^{{cite journal|author=Kittel, C.|title=Ultrasonic Propagation in Liquids|journal=J. Chem. Phys.|volume=14|issue=10|page=614|year=1946|doi=10.1063/1.1724073|bibcode = 1946JChPh..14..614K |hdl=1721.1/5041}}
47. ^{{cite journal|author=Kittel, C.|title=Interpretation of the Thermal Conductivity of Glasses|journal=Phys. Rev.|volume=75|issue=6|page=972|year=1949|doi=10.1103/PhysRev.75.972|bibcode = 1949PhRv...75..972K }}
48. ^{{cite journal|doi=10.1016/0022-3093(85)90256-X|title=Orientational ordering of local shear stresses in liquids: A phase transition?|year=1985|last1=Chen|first1=Shao-Ping|last2=Egami|first2=T.|last3=Vitek|first3=V.|journal=Journal of Non-Crystalline Solids|volume=75|issue=1–3|pages=449|bibcode = 1985JNCS...75..449C }}
49. ^{{cite journal |author=Mott, N. F. |title=The Resistance of Liquid Metals |journal=Proceedings of the Royal Society A |volume=146 |issue=857 |page=465 |year=1934 |doi=10.1098/rspa.1934.0166|bibcode = 1934RSPSA.146..465M }}
50. ^{{cite journal |author=Lindemann, C. |title=On the calculation of molecular natural frequencies |journal=Phys. Z. |volume=11 |page=609 |year=1911 |doi=}}
51. ^{{cite journal|doi=10.1038/187869b0|title=Non-crystalline Structure in Solidified Gold–Silicon Alloys|year=1960|last1=Klement|first1=W.|last2=Willens|first2=R. H.|last3=Duwez|first3=POL|journal=Nature|volume=187|issue=4740|pages=869|bibcode = 1960Natur.187..869K }}
52. ^{{cite journal|doi=10.1063/1.1735777|title=Continuous Series of Metastable Solid Solutions in Silver-Copper Alloys|year=1960|last1=Duwez|first1=Pol|last2=Willens|first2=R. H.|last3=Klement|first3=W.|journal=Journal of Applied Physics|volume=31|issue=6|pages=1136|bibcode = 1960JAP....31.1136D |url=http://authors.library.caltech.edu/3755/1/DUWjap60.pdf}}
53. ^{{cite journal|doi=10.1063/1.1735778|title=Metastable Electron Compound in Ag-Ge Alloys|year=1960|last1=Duwez|first1=Pol|last2=Willens|first2=R. H.|last3=Klement|first3=W.|journal=Journal of Applied Physics|volume=31|issue=6|pages=1137|bibcode = 1960JAP....31.1137D }}
54. ^{{cite journal|pmid=17841932|year=1978|last1=Chaudhari|first1=P|last2=Turnbull|first2=D|title=Structure and properties of metallic glasses|volume=199|issue=4324|pages=11–21|doi=10.1126/science.199.4324.11|journal=Science|bibcode = 1978Sci...199...11C }}
55. ^{{cite journal |author=Chen, J. S. |title=Glassy metals |journal=Reports on Progress in Physics |volume=43 |issue=4 |page=353 |year=1980 |doi=10.1088/0034-4885/43/4/001|bibcode = 1980RPPh...43..353C }}
56. ^{{cite journal |author1=Jonson, M. |author2=Girvin, S. M. |title=Electron-Phonon Dynamics and Transport Anomalies in Random Metal Alloys |journal=Phys. Rev. Lett. |volume=43 |issue=19 |page=1447 |year=1979 |doi=10.1103/PhysRevLett.43.1447|bibcode=1979PhRvL..43.1447J}}
57. ^{{cite journal |author=Turnbull, D. |title=Amorphous Solid Formation and Interstitial Solution Behavior in Metallic Alloy System |journal=J. Phys. C |volume=35 |issue=C4 |page=C4–1 |year=1974 |doi=10.1051/jphyscol:1974401|citeseerx=10.1.1.596.7462 }}
58. ^{{cite journal |author1=Chen, H. S. |author2=Park, B. K. |title=Role of chemical bonding in metallic glasses |journal=Acta Metall. |volume=21 |issue=4 |page=395 |year=1973 |doi=10.1016/0001-6160(73)90196-X}}
59. ^{{cite journal |author1=Wang, R. |author2=Merz, D. |title=Polymorphic bonding and thermal stability of elemental noncrystalline solids |journal=Physica Status Solidi A |volume=39 |issue=2 |page=697 |year=1977 |doi=10.1002/pssa.2210390240|bibcode = 1977PSSAR..39..697W }}
External links
{{commons category|Glass-liquid transitions}}- Fragility
- VFT Eqn.
- Polymers I
- Polymers II
- Angell: Aqueous media
- DoITPoMS Teaching and Learning Package- "The Glass Transition in Polymers"
- Ruff-Fenton degradation
- Ruffhead's Edition
- Ruffhouse Records discography
- Ruffhouse Records Discography
- Ruffin (disambiguation)
- Ruffinelli
- Ruffini ending
- Ruffinihaus
- Ruffini's endings
- Ruffin, James
- Ruffino's Bakery of New Orleans
- Ruffin-Roulhac House
- Ruffinyi
- Ruffle licen
- Rufflet
- Ruffley
- Ruffman
- Ruff-necked parrot
- Ruffneck (FULL Flex)
- Ruffneck (Full Flex)
- Ruffneck (song)
- Ruffner Hall
- Ruffner House
- Ruffner Mountain Park
- Ruff 'N' Tuff