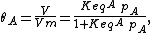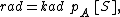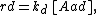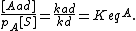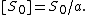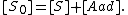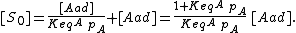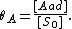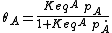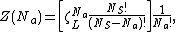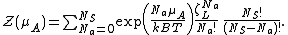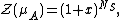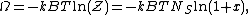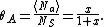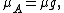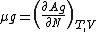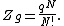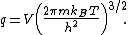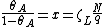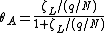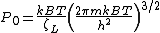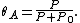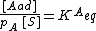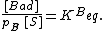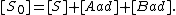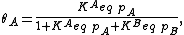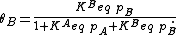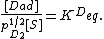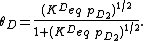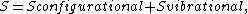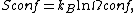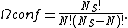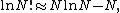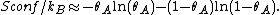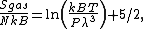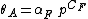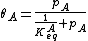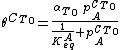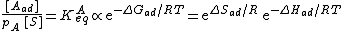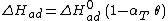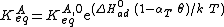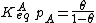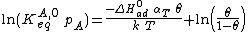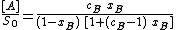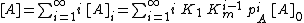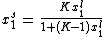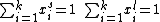- Background and experiments
- Basic assumptions of the model
- Derivations of the Langmuir adsorption isotherm Kinetic derivation Statistical mechanical derivation Competitive adsorption Dissociative adsorption
- Entropic considerations
- Disadvantages of the model
- Modifications Freundlich adsorption isotherm Temkin adsorption isotherm BET equation
- Adsorption of a binary liquid on a solid
- See also
- References
- External links
The Langmuir adsorption model explains adsorption by assuming an adsorbate behaves as an ideal gas at isothermal conditions. At these conditions the adsorbate's partial pressure, 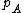 , is related to the volume of it, {{mvar|V}}, adsorbed onto a solid adsorbent. The adsorbent, as indicated in the figure, is assumed to be an ideal solid surface composed of series of distinct sites capable of binding the adsorbate. The adsorbate binding is treated as a chemical reaction between the adsorbate molecule
, is related to the volume of it, {{mvar|V}}, adsorbed onto a solid adsorbent. The adsorbent, as indicated in the figure, is assumed to be an ideal solid surface composed of series of distinct sites capable of binding the adsorbate. The adsorbate binding is treated as a chemical reaction between the adsorbate molecule 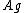 and an empty site, {{mvar|S}}. This reaction yields an adsorbed complex
and an empty site, {{mvar|S}}. This reaction yields an adsorbed complex 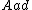 with an associated equilibrium constant
with an associated equilibrium constant 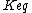 :
:
{A_{g}} + S <=> A_{ad}
From these assumptions the Langmuir isotherm can be derived (see below), which states that
where  is the fractional occupancy of the adsorption sites, and
is the fractional occupancy of the adsorption sites, and 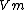 is the volume of the monolayer. A continuous monolayer of adsorbate molecules surrounding a homogeneous solid surface is the conceptual basis for this adsorption model.[1]
is the volume of the monolayer. A continuous monolayer of adsorbate molecules surrounding a homogeneous solid surface is the conceptual basis for this adsorption model.[1]
Background and experiments
In 1916, Irving Langmuir presented his model for the adsorption of species onto simple surfaces. Langmuir was awarded the Nobel Prize in 1932 for his work concerning surface chemistry. He hypothesized that a given surface has a certain number of equivalent sites to which a species can “stick”, either by physisorption or chemisorption. His theory began when he postulated that gaseous molecules do not rebound elastically from a surface, but are held by it in a similar way to groups of molecules in solid bodies.[2]
Langmuir published two papers that proved the assumption that adsorbed films do not exceed one molecule in thickness. The first experiment involved observing electron emission from heated filaments in gases.[3]
The second, a more direct proof, examined and measured the films of liquid on an adsorbent surface layer. He also noted that generally the attractive strength between the surface and the first layer of adsorbed substance is much greater than the strength between the first and second layer. However, there are instances where the subsequent layers may condense given the right combination of temperature and pressure.[4]
Basic assumptions of the model
Inherent within this model, the following assumptions[5] are valid specifically for the simplest case: the adsorption of a single adsorbate onto a series of equivalent sites on the surface of the solid.
- The surface containing the adsorbing sites is a perfectly flat plane with no corrugations (assume the surface is homogeneous) .
- The adsorbing gas adsorbs into an immobile state.
- All sites are equivalent.
- Each site can hold at most one molecule of A (mono-layer coverage only).
- There are no interactions between adsorbate molecules on adjacent sites.
Derivations of the Langmuir adsorption isotherm
Kinetic derivation
This section[5] provides a kinetic derivation for a single adsorbate case. The multiple adsorbate case is covered in the Competitive adsorption sub-section.
The model assumes adsorption and desorption as being elementary processes, where the rate of adsorption rad and the rate of desorption rd are given by
where PA is the partial pressure of A over the surface, [S] is the concentration of bare sites in number/m2, [Aad] is the surface concentration of A in molecules/m2, and kad and kd are constants of forward adsorption reaction and backward desorption reaction in the above reactions.
At equilibrium, the rate of adsorption equals the rate of desorption. Setting rad = rd and rearranging, we obtain
The concentration of sites is given by dividing the total number of sites (S_0) by the area of the adsorbate (a):
We can then calculate the concentration of all sites by summing the concentration of free sites [S] and occupied sites:
Combining this with the equilibrium equation, we get
We define now the fraction of the surface sites covered with A, θA, as
This, applied to the previous equation that combined site balance and equilibrium, yields the Langmuir adsorption isotherm:
Statistical mechanical derivation
This derivation[6][7]
was originally provided by Volmer and Mahnert[8] in 1925. The partition function of the finite number of adsorbents adsorbed on a surface, in a canonical ensemble, is given by
where 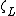 is the partition function of a single adsorbed molecule,
is the partition function of a single adsorbed molecule, 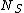 is the number of sites available for adsorption, and
is the number of sites available for adsorption, and 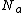 is the number of molecules adsorbed and should be less than or equal to
is the number of molecules adsorbed and should be less than or equal to 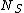 . The terms in the bracket give the total partition function of the
. The terms in the bracket give the total partition function of the 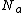 adsorbed molecules by taking a product of the individual partition functions (refer to Partition function of subsystems). The
adsorbed molecules by taking a product of the individual partition functions (refer to Partition function of subsystems). The 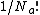 factor accounts for the overcounting arising due to the indistinguishable nature of the adsorbates. The grand canonical partition function is given by
factor accounts for the overcounting arising due to the indistinguishable nature of the adsorbates. The grand canonical partition function is given by
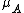 is the chemical potential of adsorbed molecule. As it has the form of binomial series, the summation is reduced to
is the chemical potential of adsorbed molecule. As it has the form of binomial series, the summation is reduced to
where 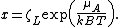
The grand potential is
based on which the average number of occupied sites is calculated
which gives the coverage
Now, invoking the condition that the system is in equilibrium, that is, the chemical potential of the adsorbed molecules is equal to that of the molecules in gas phase, we have
The chemical potential of an ideal gas is
where 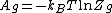 is the Helmholtz free energy of an ideal gas with its partition function
is the Helmholtz free energy of an ideal gas with its partition function
 is the partition function of a single particle in the volume of
is the partition function of a single particle in the volume of  (only consider the translational freedom here).
(only consider the translational freedom here).
We thus have 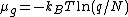 .
.
Plugging 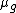 to the expression of
to the expression of  , we have
, we have
which gives the coverage
By defining
and using the identity 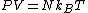 , finally, we have
, finally, we have
It is plotted in the figure alongside demonstrating that the surface coverage increases quite rapidly with the partial pressure of the adsorbants, but levels off after P reaches P0.
Competitive adsorption
The previous derivations assumes that there is only one species, A, adsorbing onto the surface. This section[9] considers the case when there are two distinct adsorbates present in the system. Consider two species A and B that compete for the same adsorption sites. The following assumptions are applied here:
- All the sites are equivalent.
- Each site can hold at most one molecule of A or one molecule of B, but not both.
- There are no interactions between adsorbate molecules on adjacent sites.
As derived using kinetical considerations, the equilibrium constants for both A and B are given by
and
The site balance states that the concentration of total sites [S0] is equal to the sum of free sites, sites occupied by A and sites occupied by B:
Inserting the equilibrium equations and rearranging in the same way we did for the single-species adsorption, we get similar expressions for both θA and θB:
Dissociative adsorption
The other case of special importance is when a molecule D2 dissociates into two atoms upon adsorption.[9] Here, the following assumptions would be held to be valid:
- D2 completely dissociates to two molecules of D upon adsorption.
- The D atoms adsorb onto distinct sites on the surface of the solid and then move around and equilibrate.
- All sites are equivalent.
- Each site can hold at most one atom of D.
- There are no interactions between adsorbate molecules on adjacent sites.
Using similar kinetic considerations, we get
The 1/2 exponent on pD2 arises because one gas phase molecule produces two adsorbed species. Applying the site balance as done above,
Entropic considerations
The formation of Langmuir monolayers by adsorption onto a surface dramatically reduces the entropy of the molecular system. This conflicts with the second law of thermodynamics, which states that entropy will increase in an isolated system. This implies that either another locally active force is stronger than the thermodynamic potential, or that our expression of the entropy of the system is incomplete.
To find the entropy decrease, we find the entropy of the molecule when in the adsorbed condition.[10]
Using Stirling's approximation, we have
On the other hand, the entropy of a molecule of an ideal gas is
where  is the thermal de Broglie wavelength of the gas molecule.
is the thermal de Broglie wavelength of the gas molecule.
Disadvantages of the model
The Langmuir adsorption model deviates significantly in many cases, primarily because it fails to account for the surface roughness of the adsorbent. Rough inhomogeneous surfaces have multiple site-types available for adsorption, with some parameters varying from site to site, such as the heat of adsorption. Moreover, specific surface area is a scale dependent quantity and no single true value exists for this parameter.[1] Thus, the use of alternative probe molecules will often result in different obtained numerical values for surface area, rendering comparison problematic.
The model also ignores adsorbate/adsorbate interactions. Experimentally, there is clear evidence for adsorbate/adsorbate interactions in heat of adsorption data. There are two kinds of adsorbate/adsorbate interactions: direct interaction and indirect interaction. Direct interactions are between adjacent adsorbed molecules, which could make adsorbing near another adsorbate molecule more or less favorable and greatly affects high-coverage behavior. In indirect interactions, the adsorbate changes the surface around the adsorbed site, which in turn affects the adsorption of other adsorbate molecules nearby.
Modifications
The modifications try to account for the points mentioned in above section like surface roughness, inhomogeneity, and adsorbate-adsorbate interactions.
Freundlich adsorption isotherm
{{main|Freundlich equation}}The Freundlich isotherm is the most important multisite adsorption isotherm for rough surfaces.
where αF and CF are fitting parameters.[11] This equation implies that if one makes a log-log plot of adsorption data, the data will fit a straight line. The Freundlich isotherm has two parameters while Langmuir's equations has only one: as a result, it often fits the data on rough surfaces better than the Langmuir's equations.
A related equation is the Toth equation. Rearranging the Langmuir equation, one can obtain:
Toth[12] modified this equation by adding two parameters, αT0 and CT0 to formulate the Toth equation:
Temkin adsorption isotherm
This isotherm takes into account indirect adsorbate-adsorbate interactions on adsorption isotherms. Temkin[13] noted experimentally that heats of adsorption would more often decrease than increase with increasing coverage.
The heat of adsorption ΔHad is defined as:
He derived a model assuming that as the surface is loaded up with adsorbate, the heat of adsorption of all the molecules in the layer would decrease linearly with coverage due to adsorbate-adsorbate interactions:
where αT is a fitting parameter. Assuming the Langmuir Adsorption isotherm still applied to the adsorbed layer, 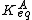 is expected to vary with coverage, as follows:
is expected to vary with coverage, as follows:
Langmuir's isotherm can be rearranged to this form:
Substituting the expression of the equilibrium constant and taking the natural logarithm:
BET equation
{{main|BET theory}}Brunauer, Emmett and Teller[14] derived the first isotherm for multilayer adsorption. It assumes a random distribution of sites that are empty or that are covered with by one monolayer, two layers and so on, as illustrated alongside. The main equation of this model is:
where
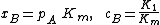
and [A] is the total concentration of molecules on the surface, given by:
where

in which [A]0 is the number of bare sites, and [A]i is the number of surface sites covered by i molecules.
Adsorption of a binary liquid on a solid
{{Main| Surface excess isotherm}}This section describes the surface coverage when the adsorbate is in liquid phase and is a binary mixture[15]
For ideal both phases - no lateral interactions, homogeneous surface - the composition of a surface phase for a binary liquid system in contact with solid surface is given by a classic Everett isotherm equation (being a simple analogue of Langmuir equation), where the components are interchangeable (i.e. "1" may be exchanged to "2") without change of eq. form:
where the normal definition of multicomponent system is valid as follows :
By simple rearrangement, we get
This equation describes competition of components "1" and "2".
See also
- Reactions on surfaces
- Hill equation (biochemistry)
References
2. ^{{cite journal|last=Langmuir|first=Irving|title=The Adsorption of Gases on Plane Surface of Glass, Mica and Platinum|journal=The Research Laboratory of the General Electric Company|date=June 1918|pages=1361–1402|doi=10.1021/ja02242a004|volume=40|issue=9}}
3. ^{{cite journal|last=Langmuir|first=Irving|title=Part I|journal=The Research Laboratory of the General Electric Company|year=1916|pages=2221|accessdate=}}
4. ^{{cite journal|last=Langmuir|first=Irving|title=Part II|journal=The Research Laboratory of the General Electric Company|year=1918|pages=1848|accessdate=}}
5. ^1 {{cite book |author=Masel, Richard | title = Principles of Adsorption and Reaction on Solid Surfaces | publisher = Wiley Interscience | year = 1996 | location = | pages = 240 | isbn = 978-0-471-30392-3}}
6. ^{{cite book |author=Masel, Richard | title = Principles of Adsorption and Reaction on Solid Surfaces | publisher = Wiley Interscience | year = 1996 | location = | pages = 242 | isbn = 978-0-471-30392-3}}
7. ^{{cite web | last = Cahill | first = David | authorlink = | title = Lecture Notes 5 Page 2 | website = | publisher = University of Illinois, Urbana Champaign | year = 2008 | url = http://www.library.uiuc.edu/ereserves/item.asp?id=35598 | format = pdf | doi = | accessdate = 2008-11-09}}
8. ^{{cite journal | last1 = Volmer | first1 = M. A. | last2 = Mahnert | first2 = P. | year = 1925 | title = Solution of Solid Substances in Liquid Surfaces and the Characteristics of Layers Thus Produced | journal = Z. Phys. Chem. | volume = 115 | issue = | page = 253 }}
9. ^1 {{cite book | author = Masel, Richard | title = Principles of Adsorption and Reaction on Solid Surfaces | publisher = Wiley Interscience | year = 1996 | location = | pages = 244 | isbn = 978-0-471-30392-3}}
10. ^{{cite web | last = Cahill | first = David | authorlink = | title = Lecture Notes 5 Page 13 | website = | publisher = University of Illinois, Urbana Champaign | year = 2008 | url = http://www.library.uiuc.edu/ereserves/item.asp?id=35598 | format = pdf | doi = | accessdate = 2008-11-09}}
11. ^{{cite journal|last1 = Freundlich|first1 = H.|journal = Kapillarchemie|date = 1909|title = eine darstellung der chemie der kolloide und verwanter gebiete.}}
12. ^{{cite journal | last1 = Toth | first1 = J | year = 1971 | title = State equations of the solid gas interface layer | journal = Acta Chim. Acad. Sci. Hung. | volume = 69 | issue = | page = 311 }}
13. ^{{cite journal|last1=Temkin|first1=M. I.|last2=Pyzhev|first2=V.|journal=Acta Physicochima USSR|date=1940|volume=12|page=327}}
14. ^{{Cite journal| doi = 10.1021/ja01269a023| issn = 0002-7863| volume = 60| issue = 2| pages = 309–319| last1 = Brunauer| first1 = Stephen| last2 = Emmett| first2 = P. H.| last3 = Teller| first3 = Edward| title = Adsorption of Gases in Multimolecular Layers| journal = Journal of the American Chemical Society| date = 1938}}
15. ^{{cite web | last = Marczewski | first = A. W | authorlink = Adam.Marczewski@umcs.lublin.pl | title = Basics of Liquid Adsorption | website = | publisher = | year = 2002 | url = http://www.adsorption.org/awm/ads/Basics.htm#Liquid | doi = | accessdate = 2008-11-24}}
- The constitution and fundamental properties of solids and liquids. part i. solids. Irving Langmuir; J. Am. Chem. Soc. 38, 2221-95 1916
External links
- Langmuir isotherm from Queen Mary, University of London
- LMMpro, Langmuir equation-fitting software
- Newroz as celebrated by Kurds in Turkey
- Newroz celebration by Kurds
- New Rules
- New rules for boats
- New Rules For Boats
- New Rules for Boats
- New Russia Township, Lorain County, Ohio
- New Russia Township, Ohio
- New Rusyn Times
- Newry and Armagh (Assembly constituency)
- Newry and Armagh (constituency)
- Newry & Armagh (Assembly constituency)
- Newry & Armagh by-election, 1986
- Newry bosco
- Newry Bosco GFC
- Newry Canal
- Newry City
- Newry City Hall
- Newry Democrat
- Newry High School
- Newry (Irish Parliament constituency)
- Newry Mitchell's GFC
- Newry Mitchel's GFC
- Newry mortar attack
- Newry, Ontario