(万历)余杭县志
浙江地方志。明戴日强纂。十卷。日强,安徽蒙城人。官余杭令。是编成于万历四十四年(1616),约六万字。分十门,六十二子目。体例冗复,如第一卷有山川门,第九卷又立径山志,既有古迹门,又别立洞霄志。记载失当之处,如余杭汉时属会稽郡,而沿革门竟记汉高帝时属荆吴国。城墉门记故城在县溪南,不详所始;不知咸淳《临安志》载汉熹平二年改, 经两次迁移,至后唐时名为清平军。诸如此类,不胜举例。《四库提要》评其“殊为疏于考订”。然为其地现存第一部志书,保存的政治、经济、文化史料亦较珍贵。有万历四十四年刊本见藏北京图书馆。
余杭县志
❶八卷。清张思齐纂修。张思齐,奉天辽阳(今辽宁辽阳)人,康熙七年(1668)知余杭县,在任时重视水利事业,开浚溪流,引溉田亩,昼夜在工地察巡,不辞劳苦。在任六年,百姓祥和。《余杭县志》康熙十二年(1673)刻本,共八卷。分为:卷一志舆地、卷二志版籍、卷三志规制、卷四志官师、卷五志选举、卷六志人物、卷七志艺文、卷八志杂志。此志基本上承袭康熙四年宋吉士志而作,宋志采辑明代成化、嘉靖、万历三志,参考《浙江通志》等书,广搜博采而成。此志在宋吉士志基础上拾遗纠误,删繁补漏。综观全书,简明扼要,言简意赅。现有清康熙二十四年(1685)重印本。
❷ 四十卷。清张吉安主修,朱文藻纂,崔应榴、董作栋续纂。张吉安,字迪民,江苏吴县(今苏州)人,曾任乾隆四十二年 (1777)举人,大挑知县,旋迁浙江,署杭州,历署临安、象山、新城、永康、丽水、浦江知县。张吉安莅任后,嘉庆十年(1805),余杭水灾,米价倍增,下令开仓卖粮,又请地方官支援大米五千石。次年又遭水害,设厂煮粥,发给百姓。后因患病,于道光九年(1829)卒。余杭士民奉其像以祀之。朱文藻(1735—1806)字映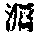 ,清浙江仁和 (今杭州市) 人。嘉庆十年 (1805),纂 《余杭县志》,未成即逝。《余杭县志》》嘉庆十年(1805)修,稿本,考余杭自宋代已有志,明代各志亦不常见。清康熙间有宋吉士志、张思齐志、龚荣志。此志体例基本承袭,龚志条目颇为详备,各图幅亦明晰。余杭山清水秀,故纪载山水、水利尤详。现有清嘉庆十三年(1808)刻本,清光绪六年(1880)王崧辰活字本和民国八年(1919)吴兰孙铅印本。
,清浙江仁和 (今杭州市) 人。嘉庆十年 (1805),纂 《余杭县志》,未成即逝。《余杭县志》》嘉庆十年(1805)修,稿本,考余杭自宋代已有志,明代各志亦不常见。清康熙间有宋吉士志、张思齐志、龚荣志。此志体例基本承袭,龚志条目颇为详备,各图幅亦明晰。余杭山清水秀,故纪载山水、水利尤详。现有清嘉庆十三年(1808)刻本,清光绪六年(1880)王崧辰活字本和民国八年(1919)吴兰孙铅印本。