- Formula
- Examples
- See also
- References
Photon energy is the energy carried by a single photon. The amount of energy is directly proportional to the photon's electromagnetic frequency and inversely proportional to the wavelength. The higher the photon's frequency, the higher its energy. Equivalently, the longer the photon's wavelength, the lower its energy.
Photon energy is solely a function of the photon's wavelength. Other factors, such as the intensity of the radiation, do not affect photon energy. In other words, two photons of light with the same color and therefore, same frequency, will have the same photon energy, even if one was emitted from a wax candle and the other from the Sun.
Photon energy can be represented by any unit of energy. Among the units commonly used to denote photon energy are the electronvolt (eV) and the joule (as well as its multiples, such as the microjoule). As one joule equals 6.24 × 1018 eV, the larger units may be more useful in denoting the energy of photons with higher frequency and higher energy, such as gamma rays, as opposed to lower energy photons, such as those in the radiofrequency region of the electromagnetic spectrum.
Formula
The equation for photon energy[1] is
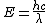
Where E is photon energy, h is the Planck constant, c is the speed of light in vacuum and λ is the photon's wavelength. As h and c are both constants, photon energy changes in inverse relation to wavelength λ.
To find the photon energy in electronvolts, using the wavelength in micrometres, the equation is approximately
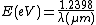
Therefore, the photon energy at 1 μm wavelength, the wavelength of near infrared radiation, is approximately 1.2398 eV.
Since 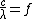 , where f is frequency, the photon energy equation can be simplified to
, where f is frequency, the photon energy equation can be simplified to

This equation is known as the Planck-Einstein relation. Substituting h with its value in J⋅s and f with its value in hertz gives the photon energy in joules. Therefore, the photon energy at 1 Hz frequency is 6.62606957 × 10−34 joules or 4.135667516 × 10−15 eV.
In chemistry and optical engineering,
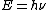
is used where h is Planck's constant and the Greek letter ν (nu) is the photon's frequency.[2]
Examples
An FM radio station transmitting at 100 MHz emits photons with an energy of about 4.1357 × 10−7 eV. This minuscule amount of energy is approximately 8 × 10−13 times the electron's mass (via mass-energy equivalence).
The highest energy gamma rays detected to date, very-high-energy gamma rays, have photon energies of 100 GeV to 100 TeV (1011 to 1014 electronvolts) or 16 nanojoules to 16 microjoules. This corresponds to frequencies of 2.42 × 1025 to 2.42 × 1028 Hz.
A photon with a wavelength equal to the Planck length would have an energy of about 7.671 × 1028 eV or 1.229 × 1010 joules (12.29 gigajoules). This is roughly the amount of energy produced by the world's most powerful coal-fired power station, the Taichung Power Plant, during a period of 2.25 seconds.
During photosynthesis, specific chlorophyll molecules absorb red-light photons at a wavelength of 700 nm in the photosystem I, corresponding to an energy of each photon of ≈ 2 eV ≈ 3 x 10−19 J ≈ 75 kBT, where kBT denotes the thermal energy. A minimum of 48 photons is needed for the synthesis of a single glucose molecule from CO2 and water (chemical potential difference 5 x 10−17 J) with a maximal energy conversion efficiency of 35%.
See also
- Photon
- Electromagnetic radiation
- Electromagnetic spectrum
- Planck constant and Planck units
- Planck–Einstein relation
References
2. ^{{cite book|author=Andrew Liddle|title=An Introduction to Modern Cosmology|url=https://books.google.com/books?id=6n64CAAAQBAJ&pg=PA16|date=27 April 2015|publisher=John Wiley & Sons|isbn=978-1-118-69025-3|pages=16}}
- Clarion-Goldfield Schools
- Clarion Hardy
- Clarion Hotel, Limerick
- Clarion Hotel The Hub (Oslo)
- Clarion Housing Group
- Clarion Inn
- ClarionProject
- Clarion School
- Clarion School, Dubai
- Clarion State Golden Eagles
- Clarion station
- Clarisa Belén Huber
- Clarisa Hardy
- Claris Airport
- Clarisa Sagardia
- Clarisa Sagardía
- Clarisa (telenovela)
- Clarisa (TV series)
- Claris (band)
- Clariscan
- Clarise Brimmer Whipple
- Clarise Fong / Blink
- Clarissa Archibald Dennis
- Clarissa Armstrong
- Clarissa Blank