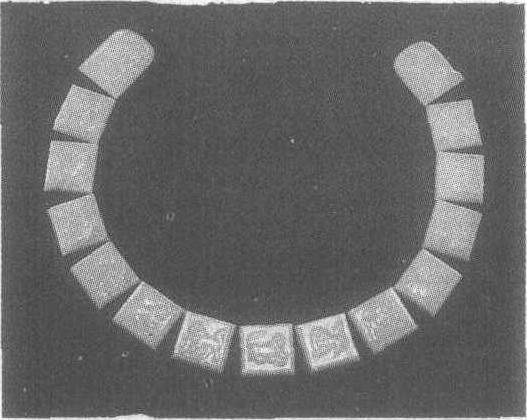
兽纹玉带板
唐。长3.5—5厘米。1970陕西省西安市何家村窖藏出土,陕西省博物馆藏。带板又名銙,缀在腰带上做为饰件。《唐实录》载:“高祖始定腰带之制。自天子以至诸侯、王、公、卿、将、相二品以上许用玉带。”由此可知,唐代开始实行玉带之制,一直延续到明代。此副玉带板为青玉所制,保存完整,包括十三块方形胯和两块铊尾。按唐代制度,三品以上人员才能着十三銙的玉带。每块板上均以浅浮雕饰一动物,并以阴线加饰细部,动物的形象各不相同,或立、或卧、或走,个个生动。每块皆有钉孔供结扎。这种雕饰动物的玉带板极为罕见。