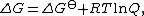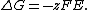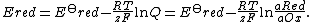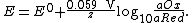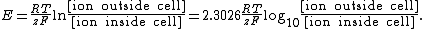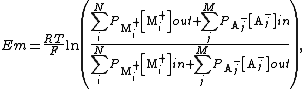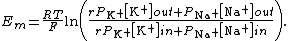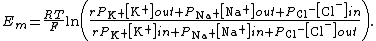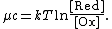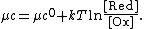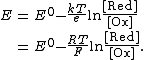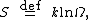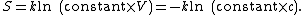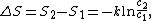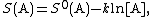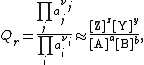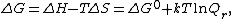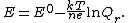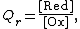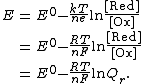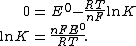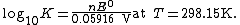- Expression
- Nernst potential
- Derivation Using Boltzmann factors Using thermodynamics (chemical potential)
- Relation to equilibrium
- Limitations Time dependence of the potential
- Significance to related scientific domains
- See also
- References
- External links
In electrochemistry, the Nernst equation is an equation that relates the reduction potential of an electrochemical reaction (half-cell or full cell reaction) to the standard electrode potential, temperature, and activities (often approximated by concentrations) of the chemical species undergoing reduction and oxidation. It was named after Walther Nernst, a German physical chemist who formulated the equation.[1][2]
Expression
The Nernst equation is derived from the standard changes in the Gibbs free energy associated with an electrochemical transformation. For any electrochemical reduction reaction of the form
Ox + z e− → Red
standard thermodynamics says that the actual free energy change {{math|ΔG}} is related to the free energy change under standard state {{math|ΔGo}} by the relationship
where {{mvar|Q}} is the reaction quotient. The electrochemical potential {{mvar|E}} associated with the electrochemical reaction is defined as the decrease in Gibbs free energy per coulomb of charge transferred, which leads to the relationship
The constant {{mvar|F}} (the Faraday constant) is a unit conversion factor {{math|F {{=}} NAq}}, where {{math|NA}} is Avogadro's number and {{mvar|q}} is the fundamental electron charge. This immediately leads to the Nernst equation.
The Nernst equation for an electrochemical half-cell is
For a complete electrochemical reaction (full cell), the equation can also be written as
(total cell potential)
where
{{math|Ered}} is the half-cell reduction potential at the temperature of interest,
{{math|E{{su|p=o|b=red}}}} is the standard half-cell reduction potential,
{{math|Ecell}} is the cell potential (electromotive force) at the temperature of interest,
{{math|E{{su|p=o|b=cell}}}} is the standard cell potential,
{{mvar|R}} is the universal gas constant: {{math|R {{=}} {{val|8.314472|(15)|u=J K−1 mol−1}}}},
{{mvar|T}} is the temperature in kelvins,
{{mvar|a}} is the chemical activity for the relevant species, where {{math|aRed}} is the activity of the reduced form and {{math|aOx}} is the activity of the oxidized form. Similarly to equilibrium constants, activities are always measured with respect to the standard state (1 mol/L for solutes, 1 atm for gases). The activity of species x, {{math|aX}} , can be related to the physical concentrations {{math|cX}} via {{math|aX {{=}} γXcX}}, where {{math|γX}} is the activity coefficient of species X. Because activity coefficients tend to unity at low concentrations, activities in the Nernst equation are frequently replaced by simple concentrations.
{{mvar|F}} is the Faraday constant, the number of coulombs per mole of electrons: {{math|F {{=}} {{val|9.64853399|(24)|e=4|u=C mol−1}}}},
{{mvar|z}} is the number of electrons transferred in the cell reaction or half-reaction,
{{math|Qr}} is the reaction quotient of the cell reaction.
At room temperature (25 °C), {{math|{{sfrac|RT|F}}}} may be treated as a constant and replaced by 25.693 mV for cells.
The Nernst equation is frequently expressed in terms of base-10 logarithms (i.e., common logarithms) rather than natural logarithms, in which case it is written, for a cell at 25 °C:
The Nernst equation is used in physiology for finding the electric potential of a cell membrane with respect to one type of ion.
Nernst potential
{{main|Reversal potential}}The Nernst equation has a physiological application when used to calculate the potential of an ion of charge {{math|z}} across a membrane. This potential is determined using the concentration of the ion both inside and outside the cell:
When the membrane is in thermodynamic equilibrium (i.e., no net flux of ions), the membrane potential must be equal to the Nernst potential. However, in physiology, due to active ion pumps, the inside and outside of a cell are not in equilibrium. In this case, the resting potential can be determined from the Goldman equation, which is a solution of G-H-K influx equation under the constraints that total current density driven by electrochemical force is zero:
where
{{math|Em}} is the membrane potential (in volts, equivalent to joules per coulomb),
{{math|Pion}} is the permeability for that ion (in meters per second),
{{math|[ion]out}} is the extracellular concentration of that ion (in moles per cubic meter, to match the other SI units, though the units strictly don't matter, as the ion concentration terms become a dimensionless ratio),
{{math|[ion]in}} is the intracellular concentration of that ion (in moles per cubic meter),
{{mvar|R}} is the ideal gas constant (joules per kelvin per mole),
{{mvar|T}} is the temperature in kelvins,
{{mvar|F}} is Faraday's constant (coulombs per mole).
The potential across the cell membrane that exactly opposes net diffusion of a particular ion through the membrane is called the Nernst potential for that ion. As seen above, the magnitude of the Nernst potential is determined by the ratio of the concentrations of that specific ion on the two sides of the membrane. The greater this ratio the greater the tendency for the ion to diffuse in one direction, and therefore the greater the Nernst potential required to prevent the diffusion.
A similar expression exists that includes {{mvar|r}} (the absolute value of the transport ratio). This takes transporters with unequal exchanges into account. See: sodium-potassium pump where the transport ratio would be 2/3, so r equals 1.5 in the formula below. The reason why we insert a factor r = 1.5 here is that current density by electrochemical force Je.c.(Na+)+Je.c.(K+) is no longer zero, but rather Je.c.(Na+)+1.5Je.c.(K+)=0 (as for both ions flux by electrochemical force is compensated by that by the pump, i.e. Je.c.=-Jpump), altering the constraints for applying GHK equation. The other variables are the same as above. The following example includes two ions: potassium (K+) and sodium (Na+). Chloride is assumed to be in equilibrium.
When chloride (Cl−) is taken into account,
Derivation
Using Boltzmann factors
For simplicity, we will consider a solution of redox-active molecules that undergo a one-electron reversible reaction
Ox + e− {{eqm}} Red
and that have a standard potential of zero. The chemical potential {{math|μc}} of this solution is the difference between the energy barriers for taking electrons from and for giving electrons to the working electrode that is setting the solution's electrochemical potential.
The ratio of oxidized to reduced molecules, {{sfrac|[Ox]|[Red]}}, is equivalent to the probability of being oxidized (giving electrons) over the probability of being reduced (taking electrons), which we can write in terms of the Boltzmann factor for these processes:
Taking the natural logarithm of both sides gives
If {{math|μc ≠ 0}} at {{sfrac|[Ox]|[Red]}} = 1, we need to add in this additional constant:
Dividing the equation by {{mvar|e}} to convert from chemical potentials to electrode potentials, and remembering that {{math|{{sfrac|k|e}} {{=}} {{sfrac|R|F}}}},[3] we obtain the Nernst equation for the one-electron process {{nowrap|Ox + e− → Red}}:
Using thermodynamics (chemical potential)
Quantities here are given per molecule, not per mole, and so Boltzmann constant {{math|k}} and the electron charge {{math|e}} are used instead of the gas constant {{math|R}} and Faraday's constant {{math|F}}. To convert to the molar quantities given in most chemistry textbooks, it is simply necessary to multiply by Avogadro's number: {{math|R {{=}} kNA}} and {{math|F {{=}} eNA}}.
The entropy of a molecule is defined as
where {{math|Ω}} is the number of states available to the molecule. The number of states must vary linearly with the volume {{math|V}} of the system (here an idealized system is considered for better understanding, so that activities are posited very close to the true concentrations. Fundamental statistical proof of the mentioned linearity goes beyond the scope of this section, but to see this is true it is simpler to consider usual isothermal process for an ideal gas where the change of entropy {{math|ΔS {{=}} nR ln({{sfrac|V2|V1}})}} takes place. It follows from the definition of entropy and from the condition of constant temperature and quantity of gas {{mvar|n}} that the change in the number of states must be proportional to the relative change in volume {{math|{{sfrac|V2|V1}}}}. In this sense there is no difference in statistical properties of ideal gas atoms compared with the dissolved species of a solution with activity coefficients equaling one: particles freely "hang around" filling the provided volume), which is inversely proportional to the concentration {{mvar|c}}, so
we can also write the entropy as
The change in entropy from some state 1 to another state 2 is therefore
so that the entropy of state 2 is
If state 1 is at standard conditions, in which {{math|c1}} is unity (e.g., 1 atm or 1 M), it will merely cancel the units of {{math|c2}}. We can, therefore, write the entropy of an arbitrary molecule A as
where {{math|S0}} is the entropy at standard conditions and [A] denotes the concentration of A.
The change in entropy for a reaction
{{mvar|a}} A + {{mvar|b}} B → {{mvar|y}} Y + {{mvar|z}} Z
is then given by
We define the ratio in the last term as the reaction quotient:
where the numerator is a product of reaction product activities, {{math|aj}}, each raised to the power of a stoichiometric coefficient, {{math|νj}}, and the denominator is a similar product of reactant activities. All activities refer to a time {{math|t}}. Under certain circumstances (see chemical equilibrium) each activity term such as {{math|a{{su|b=j|p=νj}}}} may be replaced by a concentration term, [A].
In an electrochemical cell, the cell potential {{math|E}} is the chemical potential available from redox reactions ({{math|E {{=}} {{sfrac|μc|e}}}}). {{math|E}} is related to the Gibbs energy change {{math|ΔG}} only by a constant:
{{math|ΔG {{=}} −nFE}}, where {{math|n}} is the number of electrons transferred and {{math|F}} is the Faraday constant. There is a negative sign because a spontaneous reaction has a negative free energy {{math|ΔG}} and a positive potential {{math|E}}. The Gibbs energy is related to the entropy by {{math|G {{=}} H − TS}}, where {{math|H}} is the enthalpy and {{math|T}} is the temperature of the system. Using these relations, we can now write the change in Gibbs energy,and the cell potential,
This is the more general form of the Nernst equation. For the redox reaction {{nowrap|Ox + {{mvar|n}} e− → Red}},
and we have:
The cell potential at standard conditions {{math|E0}} is often replaced by the formal potential {{math|E0′}}, which includes some small corrections to the logarithm and is the potential that is actually measured in an electrochemical cell.
Relation to equilibrium
At equilibrium, the electrochemical potential {{math|E {{=}} 0}} and therefore the reaction quotient attains the special value known as the equilibrium constant: {{math|Q {{=}} K}}. Therefore,
Or at standard temperature,
We have thus related the standard electrode potential and the equilibrium constant of a redox reaction.
Limitations
In dilute solutions, the Nernst equation can be expressed directly in the terms of concentrations (since activity coefficients are close to unity). But at higher concentrations, the true activities of the ions must be used. This complicates the use of the Nernst equation, since estimation of non-ideal activities of ions generally requires experimental measurements.
The Nernst equation also only applies when there is no net current flow through the electrode. The activity of ions at the electrode surface changes when there is current flow, and there are additional overpotential and resistive loss terms which contribute to the measured potential.
At very low concentrations of the potential-determining ions, the potential predicted by Nernst equation approaches toward {{math|±∞}}. This is physically meaningless because, under such conditions, the exchange current density becomes very low, and there is no thermodynamic equilibrium necessary for Nernst equation to hold. The electrode is called unpoised in such case. Other effects tend to take control of the electrochemical behavior of the system.
Time dependence of the potential
The expression of time dependence has been established by Karaoglanoff.[4][5][6][7]
Significance to related scientific domains
The equation has been involved in the scientific controversy involving cold fusion. The discoverers of cold fusion, Fleischmann and Pons, calculated that a palladium cathode immersed in a heavy water electrolysis cell could achieve up to 1027 atmospheres of pressure on the surface of the cathode, enough pressure to cause spontaneous nuclear fusion. In reality, only 10,000–20,000 atmospheres were achieved. John R. Huizenga claimed their original calculation was affected by a misinterpretation of Nernst equation.[8] He cited a paper about Pd–Zr alloys.[9]
The equation permits the extent of reaction between two redox systems to be calculated and can be used, for example, to decide whether a particular reaction will go to completion or not.
At equilibrium the emfs of the two half cells are equal. This enables {{math|Kc}} to be calculated hence the extent of the reaction.
See also
- Concentration cell
- Electrode potential
- Galvanic cell
- Goldman equation
- Membrane potential
- Nernst–Planck equation
- Solvated electron
References
2. ^{{Cite journal | last = Wahl | year = 2005 | title = A Short History of Electrochemistry | journal = Galvanotechtnik | volume = 96 | issue = 8 | pages = 1820–1828 }}
3. ^{{math|R {{=}} NAk}}; see gas constant
{{math|F {{=}} eNA}}; see Faraday constant
4. ^{{citation |first=Z. |last=Karaoglanoff |authorlink=Zakhari Karaoglanoff |title=Über Oxydations- und Reduktionsvorgänge bei der Elektrolyse von Eisensaltzlösungen |language=German |trans-title=On Oxidation and Reduction Processes in the Electrolysis of Iron Salt Solutions |journal=Zeitschrift für Elektrochemie |volume=12 |issue=1 |pages=5–16 |date=January 1906 |doi=10.1002/bbpc.19060120105 }}
5. ^{{citation |title=Electrochemical Dictionary |editor1-first=Allen J. |editor1-last=Bard |editor2-first=György |editor2-last=Inzelt |editor3-first=Fritz |editor3-last=Scholz |contribution=Karaoglanoff equation |pages=527–528 |url=https://books.google.com/?id=4TBWg3dIyKQC&pg=PA527&lpg=PA527 |publisher=Springer|isbn=9783642295515 |date=2012-10-02 }}
6. ^{{citation |title=Introduction to Polarography and Allied Techniques |first=Kamala |last=Zutshi |pages=127–128 |url=https://books.google.com/?id=WmiaCVH-MEIC&pg=PA127&lpg=PA127 |isbn= 9788122417913|year=2008}}
7. ^The Journal of Physical Chemistry, Volume 10, p 316. https://books.google.com/books?id=zCMSAAAAIAAJ&pg=PA316&lpg=PA316&hl=en&f=false
8. ^{{cite book| last=Huizenga | first=John R. | authorlink=John R. Huizenga | title=Cold Fusion: The Scientific Fiasco of the Century | edition=2 | location=Oxford and New York | publisher=Oxford University Press | year=1993 | pages=33, 47 | isbn=978-0-19-855817-0 }}
9. ^{{cite journal|last1=Huot|first1=J. Y.|title=Electrolytic Hydrogenation and Amorphization of Pd-Zr Alloys|journal=Journal of the Electrochemical Society|volume=136|issue=3|year=1989|pages=630|issn=0013-4651|doi=10.1149/1.2096700}}
External links
- Nernst/Goldman Equation Simulator
- Nernst Equation Calculator
- Interactive Nernst/Goldman Java Applet
- DoITPoMS Teaching and Learning Package- "The Nernst Equation and Pourbaix Diagrams"
- 1907 Vanderbilt Commodores football season
- 1907 Vermont Catamounts football
- 1907 Vermont Catamounts football season
- 1907 Vermont Catamounts football team
- 1907 Villanova Wildcats football
- 1907 Villanova Wildcats football season
- 1907 Villanova Wildcats football team
- 1907 Virginia Cavaliers football
- 1907 Virginia Cavaliers football season
- 1907 Virginia Cavaliers football team
- 1907 Virginia Tech Hokies football team
- 1907 VMI Keydets football
- 1907 VMI Keydets football season
- 1907 VPI football
- 1907 VPI football season
- 1907 VPI football team
- 1907 VPI Hokies football team
- 1907 WAFA ladder
- 1907 WAFA season
- 1907 Washington football
- 1907 Washington football season
- 1907 Washington & Jefferson Presidents football
- 1907 Washington & Jefferson Presidents football season
- 1907 Washington & Jefferson Presidents football team
- 1907 Washington State football