- {{anchor|Electric polarizability|Electronic polarizability}}Electric polarizability Definition Application in crystallography Tendencies
- {{anchor|Magnetic polarizability}}Magnetic polarizability
- See also
- References
{{anchor|Electric polarizability|Electronic polarizability}}Electric polarizability
Definition
Electric polarizability is the relative tendency of a charge distribution, like the electron cloud of an atom or molecule, and consequently of any material body, to have its charges displaced by any external electric field, which in the uniform case is applied typically by a charged parallel-plate capacitor.
The polarizability  in isotropic media is defined as the ratio of the induced dipole moment
in isotropic media is defined as the ratio of the induced dipole moment  of an atom to the electric field
of an atom to the electric field 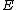 that produces this dipole moment.[3]
that produces this dipole moment.[3]
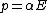
Polarizability has the SI units of C·m2·V−1 = A2·s4·kg−1 while its cgs unit is cm3. Usually it is expressed in cgs units as a so-called polarizability volume, sometimes expressed in Å3 = 10−24 cm3. One can convert from SI units to cgs units as follows:
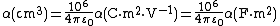 ≃ 8.988×1015 ×
≃ 8.988×1015 × 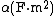
where  , the vacuum permittivity, is ~8.854 × 10−12 (F/m). If the polarizability volume is denoted
, the vacuum permittivity, is ~8.854 × 10−12 (F/m). If the polarizability volume is denoted 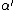 the relation can also be expressed generally[4] (in SI) as
the relation can also be expressed generally[4] (in SI) as 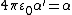 .
.
The polarizability of individual particles is related to the average electric susceptibility of the medium by the Clausius-Mossotti relation.
Polarizability for anisotropic or non-spherical media cannot in general be represented as a scalar quantity. Defining  as a scalar implies both that applied electric fields can only induce polarization components parallel to the field and that the
as a scalar implies both that applied electric fields can only induce polarization components parallel to the field and that the  and
and  directions respond in the same way to the applied electric field. For example, an electric field in the
directions respond in the same way to the applied electric field. For example, an electric field in the  -direction can only produce an
-direction can only produce an  component in
component in  and if that same electric field were applied in the
and if that same electric field were applied in the  -direction the induced polarization would be the same in magnitude but appear in the
-direction the induced polarization would be the same in magnitude but appear in the  component of
component of  . Many crystalline materials have directions that are easier to polarize than others and some even become polarized in directions perpendicular to the applied electric field, and the same thing happens with non-spherical bodies. Some molecules and materials with this sort of anisotropic behavior are often optically active, exhibiting effects such as birefringence of light.
. Many crystalline materials have directions that are easier to polarize than others and some even become polarized in directions perpendicular to the applied electric field, and the same thing happens with non-spherical bodies. Some molecules and materials with this sort of anisotropic behavior are often optically active, exhibiting effects such as birefringence of light.
To describe anisotropic media a polarizability rank two tensor or  matrix
matrix  is defined,
is defined,
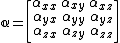
The elements describing the response parallel to the applied electric field are those along the diagonal. A large value of 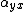 here means that an electric-field applied in the
here means that an electric-field applied in the  -direction would strongly polarize the material in the
-direction would strongly polarize the material in the  -direction. Explicit expressions for
-direction. Explicit expressions for  have been given for homogeneous anisotropic ellipsoidal bodies.[5][6]
have been given for homogeneous anisotropic ellipsoidal bodies.[5][6]
Application in crystallography
The matrix above can be used with the molar refractivity equation and other data to produce density data for crystallography. Each polarizability measurement along with the refractive index associated with its direction will yield a direction specific density that can be used to develop an accurate three dimensional assessment of molecular stacking in the crystal. This relationship was first observed by Linus Pauling.[2]
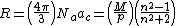
R = Molar refractivity , 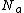 = Avogadro's number,
= Avogadro's number, 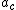 = electronic polarization, p = density, M = Molar mass, n = refractive index
= electronic polarization, p = density, M = Molar mass, n = refractive index
Tendencies
Generally, polarizability increases as the volume occupied by electrons increases.[8] In atoms, this occurs because larger atoms have more loosely held electrons in contrast to smaller atoms with tightly bound electrons.[8][7] On rows of the periodic table, polarizability therefore decreases from left to right.[8] Polarizability increases down on columns of the periodic table.[8] Likewise, larger molecules are generally more polarizable than smaller ones.
Water is a very polar molecule, but alkanes and other hydrophobic molecules are more polarizable. Water with its permanent dipole is less likely to change shape due to an external electric field. Alkanes are the most polarizable molecules.[8] Although alkenes and arenes are expected to have larger polarizability than alkanes because of their higher reactivity compared to alkanes, alkanes are in fact more polarizable.[8] This results because of alkene's and arene's more electronegative sp2 carbons to the alkane's less electronegative sp3 carbons.[8]
Ground state electron configuration models are often inadequate in studying the polarizability of bonds because dramatic changes in molecular structure occur in a reaction.[8]
{{anchor|Magnetic polarizability}}Magnetic polarizability
Magnetic polarizability defined by spin interactions of nucleons is an important parameter of deuterons and hadrons. In particular, measurement of tensor polarizabilities of nucleons yields important information about spin-dependent nuclear forces.[9]
The method of spin amplitudes uses quantum mechanics formalism to more easily describe spin dynamics. Vector and tensor polarization of particle/nuclei with spin {{math|S ≥ 1}} are specified by the unit polarization vector  and the polarization tensor P`. Additional tensors composed of products of three or more spin matrices are needed only for the exhaustive description of polarization of particles/nuclei with spin {{math|S ≥ {{frac|3|2}}}} .[9]
and the polarization tensor P`. Additional tensors composed of products of three or more spin matrices are needed only for the exhaustive description of polarization of particles/nuclei with spin {{math|S ≥ {{frac|3|2}}}} .[9]
See also
- Dielectric
- Electric susceptibility
- Polarization density
- MOSCED, an estimation method for activity coefficients; uses polarizability as parameter
References
2. ^1 {{Cite book|title=The CRC Handbook of Chemistry and Physics|last=Lide|first=David|publisher=The Chemical Rubber Publishing Company|year=1998|isbn=|location=|pages=12–17|quote=|via=}}
3. ^Introduction to Electrodynamics (3rd Edition), D.J. Griffiths, Pearson Education, Dorling Kindersley, 2007, {{ISBN|81-7758-293-3}}
4. ^{{cite book|title=Atkins' Physical Chemistry|year=2010|publisher=Oxford University Press|isbn=978-0-19-954337-3|pages=622–629|last1=Atkins|first1=Peter|last2=de Paula|first2=Julio|chapter=17}}
5. ^Electrodynamics of Continuous Media, L.D. Landau and E.M. Lifshitz, Pergamon Press, 1960, pp. 7 and 192.
6. ^C.E. Solivérez, Electrostatics and Magnetostatics of Polarized Ellipsoidal Bodies: The Depolarization Tensor Method, Free Scientific Information, 2016 (2nd edition), {{ISBN|978-987-28304-0-3}}, pp. 20, 23, 32, 30, 33, 114 and 133.
7. ^{{cite book| last1 = Schwerdtfeger | first1 = Peter | title = Atomic Static Dipole Polarizabilities | editor = G. Maroulis | chapter = Computational Aspects of Electric Polarizability Calculations: Atoms, Molecules and Clusters | publisher = IOS Press | year = 2006 }} {{dead link|date=January 2018 |bot=InternetArchiveBot |fix-attempted=yes }}
8. ^1 2 3 4 5 6 7 {{cite book| last1 = Anslyn| last2 = Dougherty| first1 = Eric| first2 = Dennis | authorlink2 = Dennis A. Dougherty | title = Modern Physical Organic Chemistry | publisher = University Science | year = 2006 | isbn = 978-1-891389-31-3}}[https://books.google.com/books?id=gY-Sxijk_tMC&pg=PA25&dq=organic+chemistry+polarizability&hl=en&ei=NasLToOwF8Ta0QG7kq2iAQ&sa=X&oi=book_result&ct=result&resnum=1&ved=0CC0Q6AEwAA#v=onepage&q=organic%20chemistry%20polarizability&f=false]
9. ^1 {{cite web |author=A. J. Silenko |title=Manifestation of tensor magnetic polarizability of the deuteron in storage ring experiments |url=http://www.springerlink.com/content/m2744373305l24m7/ |publisher=Springer Berlin / Heidelberg |date=18 Nov 2008 |doi=10.1140/epjst/e2008-00776-9 |accessdate=25 May 2010|bibcode=2008EPJST.162...59S }}
- Hayate no gotoku
- Hayate no Gotoku! (KOTOKO song)
- Hayate no Gotoku! (song)
- Hayate (Savage Reign)
- Hayate (Street Fighter)
- Hayate (Street Fighter character)
- Hayate The Combat Butler
- Hayate the combat butler
- Hayate the Combat Butler
- Hayate (train service)
- Hayateumi
- Hayateumi Hidehito
- Hayate Usui
- Hayate Yagami
- Hayate × Blade
- Hayathnagar
- Hayati (album)
- Hayat-i-Javed
- Hayati Yazıcı
- Hayat Khan Sherpao
- Hayat Mohammad Khan Sherpao
- Hayato Gokudera
- Hayato Jin
- Hayato Juumonji
- Hayato Kazami