- Koutecký–Levich plot
- References
The Koutecký–Levich equation models the measured electric current at an electrode from a electrochemical reaction in relation to the kinetic activity and the mass transport of reactants.
The Koutecký–Levich equation can be written as[1]:
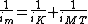
where
- im is the measured current (A).
- iK is the kinetic current (A) from the electrochemical reactions.
- iMT is the mass transport current (A).
Note the similarity of this equation to the conductance of an electrical circuits in series.
The Koutecký–Levich equation is also commonly expressed as:
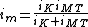
The kinetic current (iK) can be modeled by the Butler-Volmer Equation and is characterized by being potential dependent. On the other hand, the mass transport current (iMT) depends on the particular electrochemical setup and amount of stirring.
Koutecký–Levich plot
In the case a rotating disk electrode setup is used and the electrode is flat and smooth, the iMT can modeled using the Levich equation.[1][2] Inserted in the Koutecký-Levich equation, we get:
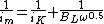
where:
- BL is the Levich Constant.
- ω is the angular rotation rate of the electrode (rad/s)
From an experimental data set where the current is measured at different rotation rates, it is possible to extract the kinetic current from a so-called Koutecký–Levich plot. In a Koutecký–Levich plot the inverse measured current is plotted versus the inverse square root of the rotation rate. This will linearize the data set and the inverse of the kinetic current can be obtained by extrapolating the line to the ordinate.
References
2. ^{{Cite book|title=Physicochemical Hydrodynamics|last=Levich|first=V. G.|publisher=Prentice-Hall|year=1962|isbn=0136744400|location=Englewood Cliffs, N.J|pages=}}
- Voice of an angel
- Voice of Asia
- Voice of Barbados
- Voice of Beslan
- Voice of Britain
- Voice of Canadians
- Voice of Citizens Together
- Voice of Croatia
- Voice of Democracy
- Voice of Dharma
- Voice of Fire
- Voice of Free China
- Voice of God
- Voice of God Collective
- Voice of Ho Chi Minh City
- Voice of India
- Voice of Israel
- Voice Of Korea
- Voice of Korea
- Voice of Korea (Montreal)
- Voice of London
- Voice of Love
- Voice of Maine News/Talk Network
- Voice Of Music
- Voice of Music