万家寨水利枢纽
黄河干流水利工程。引黄入晋工程的起点。控制流域面积39.5万km2。水库总库容8.96亿m3。坝顶长443 m,最大坝高105 m。电站装机容量108万kW,年发电量27.5亿kW·h。左岸设有2个引黄取水口,单孔引水流量24 m3/s。枢纽年供水量14亿m3,其中山西太原6.4亿m3,大同、平朔5.6亿m3,内蒙古准格尔旗2.0亿m3。1992年开始施工准备,1994年11月主体工程开工,1995年12月截流,1998年11月28日第1台机组发电,2002年竣工。
万家寨水利枢纽
一座以供水为主,结合发电调峰,兼有防洪、防凌等综合利用的大型水利枢纽工程。位于黄河北干流上段托克托至龙口峡谷河段内。坝址左岸为山西省偏关县,右岸为内蒙古自治区准格尔旗,坝址以上流域面积394813平方千米。坝址段河道比降1.24‰,河宽300米~500米。枢纽采用半整体式混凝土直线重力坝,最大坝高90米,坝顶长436米,水库总库容8.96亿立方米,调节库容4.45亿立方米。采用坝后式厂房,电站装机6台18万千瓦水轮发电机组。能为山西及内蒙古能源基地、工业和城市用水及部分农业补水,年提供水量14亿立方米。
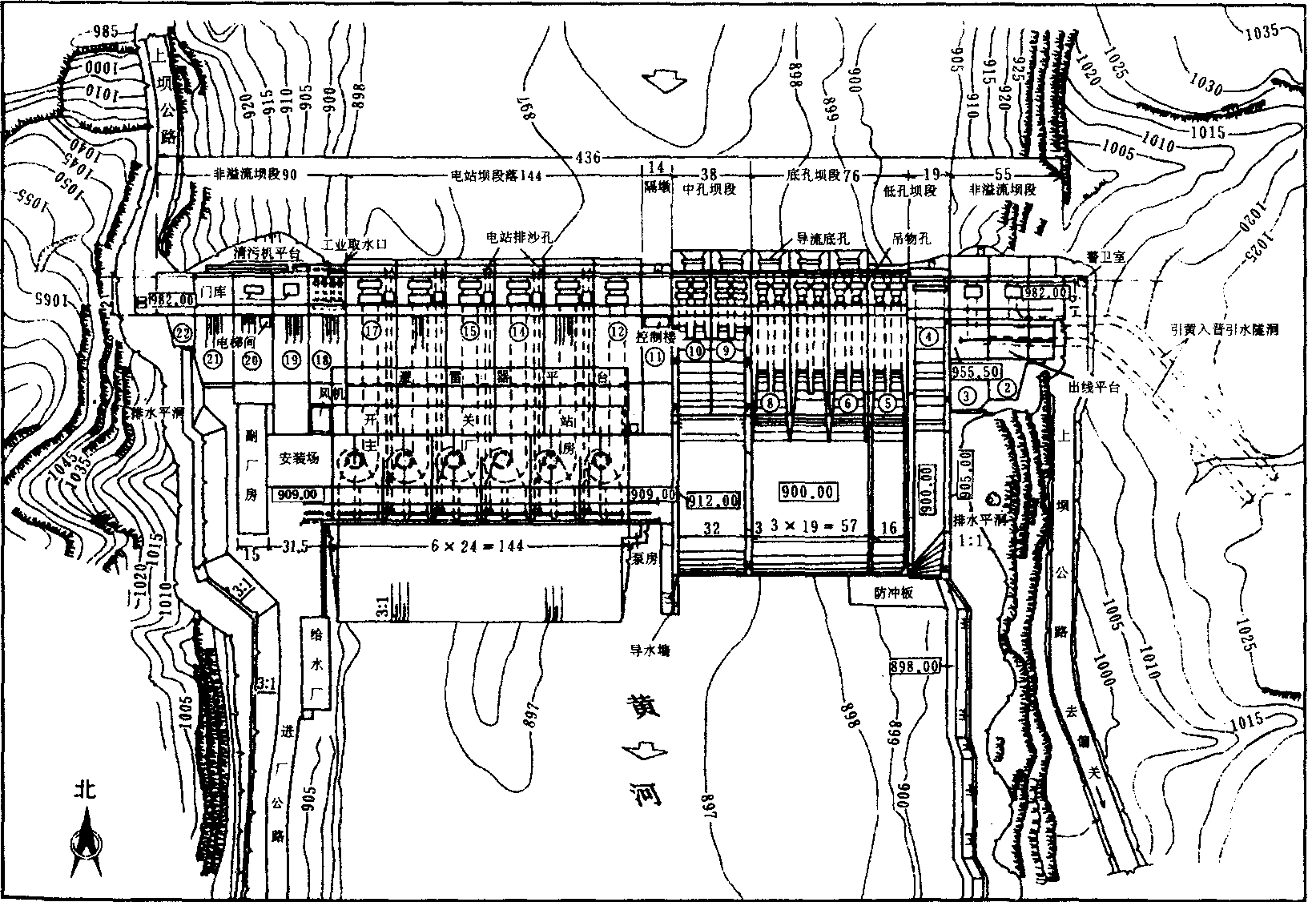
万家寨水利枢纽平面布置图
枢纽工程设计总工期为六年半,1994年底主体工程正式开工,1995年12月9日大河截流成功,1998年大坝下闸蓄水、供水发电,2000年工程将全部竣工。