焦距jiaoju
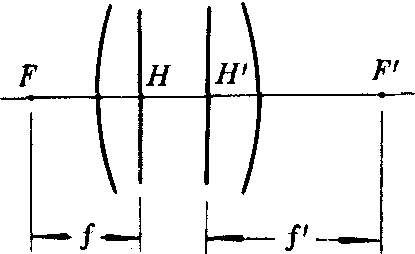
光学系统会聚或发散光束能力的一种量度。前主点H到前焦点F的距离叫做前焦距,又称物方焦距或第一焦距,常用f表示;后主点H′到后焦点F′的距离叫做后焦距,又称像方焦距或第二焦距,常用f′表示。按几何光学的符号法则,焦点在主点左方者焦距为负,焦点在主点右方者焦距为正。就图所示的情况,f<0,f′>0。f′>0者使光束会聚,f′<0者使光束发散。焦距数值越小,光学系统会聚或发散光束的能力越大。可以证明,光学系统两个焦距之比为
f′/f=n′/n
式中n和n′分别为物方折射率和像方折射率。在通常n=n′的情况下,f和f′的数值相等而符号相反。球面镜的焦距为球面顶点到焦点的距离,其值等于球面曲率半径的一半。一般说,透镜的焦距取决于两表面的曲率半径、透镜厚度、材料和周围媒质的折射率。对于放在空气中的薄透镜,其焦距为
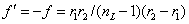
式中r1和r2分别为透镜前后两个表面的曲率半径,按几何光学的符号法则,球面曲率中心在透镜左者为负,右者为正;nL为透镜材料的折射率。
焦距
见“物理学”中的“焦距”。
焦距
曲面镜或透镜中某一点与其主焦点之间的距离。曲面镜从其顶点算起,薄透镜约从其中心算起,厚透镜和透镜组则从其主焦点所在一侧的主平面算起。
焦距
当照相机的镜头对无限远的景物聚焦时,从胶片到象方主点(位置靠近镜头光心)之间的距离称为该镜头的焦距。镜头的焦距一般都刻在镜身上,如50毫米,就是表明该镜头焦距是50毫米。又如28毫米—70毫米,就表示该镜头的焦距是从28毫米至70毫米。在被摄物与照相机之间距离不变的情况下,焦距长短影响到下列因素:
❶焦距越长,象角越小;焦距越短,象角越大。
❷焦距越长,光孔越小;焦距越短,光孔越大。
❸焦距越长,拍摄范围越小;焦距越短,拍摄范围越大。
焦距
照相机对无穷远调焦时,从镜头的光学中心到焦平面的距离。焦距近似于画幅对角线长的镜头称为标准镜头。135相机50mm焦距的镜头、120相机6×6画幅75~80mm焦距、6×9画幅105mm焦距的镜头都是典型的标准镜头。焦距比标准镜头更长的镜头称为中焦、长焦镜头,焦距比标准镜头更短的镜头称为短焦或广角镜头。