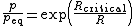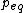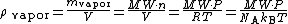- History
- Derivation from Kelvin's equation
- References
- See also
The Ostwald–Freundlich equation governs boundaries between two phases; specifically, it relates the surface tension of the boundary to its curvature, the ambient temperature, and the vapor pressure or chemical potential in the two phases.
The Ostwald–Freundlich equation for a droplet or particle with radius  is:
is:
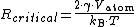
: Atomic volume
: Boltzmann constant
: Surface tension (J
m−2)
: Equilibrium partial pressure (or chemical potential or concentration)
: Partial pressure (or chemical potential or concentration)
: Absolute temperature
One consequence of this relation is that small liquid droplets (i.e., particles with a high surface curvature) exhibit a higher effective vapor pressure, since the surface is larger in comparison to the volume.
Another notable example of this relation is Ostwald ripening, in which surface tension causes small precipitates to dissolve and larger ones to grow. Ostwald ripening is thought to occur in the formation of orthoclase megacrysts in granites as a consequence of subsolidus growth. See rock microstructure for more.
History
In 1871, Lord Kelvin (William Thomson) obtained the following relation governing a liquid-vapor interface:[1]
where
: vapor pressure at a curved interface of radius
: vapor pressure at flat interface (
) =
: surface tension
: density of vapor
: density of liquid
,
: radii of curvature along the principal sections of the curved interface.
In his dissertation of 1885, Robert von Helmholtz (son of the German physicist Hermann von Helmholtz) derived the Ostwald–Freundlich equation and showed that Kelvin's equation could be transformed into the Ostwald–Freundlich equation.[2][3] The German physical chemist Wilhelm Ostwald derived the equation apparently independently in 1900;[4] however, his derivation contained a minor error which the German chemist Herbert Freundlich corrected in 1909.[5]
Derivation from Kelvin's equation
According to Lord Kelvin's equation of 1871,[6][7]
If the particle is assumed to be spherical, then.
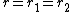 ; hence,
; hence,Note: Kelvin defined the surface tension.
 as the work that was performed per unit area by the interface rather than on the interface; hence his term containing
as the work that was performed per unit area by the interface rather than on the interface; hence his term containing  has a minus sign. In what follows, the surface tension will be defined so that the term containing
has a minus sign. In what follows, the surface tension will be defined so that the term containing  has a plus sign.
has a plus sign.Since 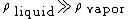 ,
,
then 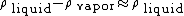 ; hence,
; hence,
Assuming that the vapor obeys the ideal gas law, then.
where
: mass of a volume
of vapor
: molecular weight of vapor
: number of moles of vapor in volume
of vapor
: Avogadro constant
: ideal gas constant =
Since 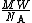 : mass of one molecule of vapor or liquid, then
: mass of one molecule of vapor or liquid, then
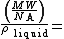 volume of one molecule
volume of one molecule 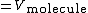 .
.
Hence
where,
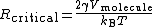 .
.Thus
Since.
then,
Since.
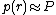 , then
, then 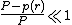 .
.If 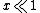 , then
, then 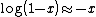 .
.
Hence
Therefore.
which is the Ostwald–Freundlich equation.,
References
2. ^Robert von Helmholtz (1886) [https://books.google.com/books?id=9xVbAAAAYAAJ&pg=PA508#v=onepage&q&f=false "Untersuchungen über Dämpfe und Nebel, besonders über solche von Lösungen"] (Investigations of vapors and mists, and especially of such things from solutions), Annalen der Physik, 263 (4) : 508-543. On pages 522-525 Helmholtz derives the Ostwald-Freundlich equation and subsequently converts Kelvin's equation into the Ostwald-Freundlich equation.
3. ^Robert von Helmholtz's derivation of the Ostwald-Freundlich equation from Kelvin's equation appears on the "Talk" page of this article.
4. ^Ostwald, W. (1900) [https://books.google.com/books?id=xTYpAQAAIAAJ&pg=PA495#v=onepage&q&f=false "Über die vermeintliche Isomerie des roten und gelben Quecksilbersoxyds und die Oberflächenspannung fester Körper"] (On the supposed isomerism of red and yellow mercury oxide and the surface tension of solid bodies) Zeitschrift für physikalische Chemie, 34 : 495-503. Ostwald’s equation relating temperature, solubility, surface tension, and the radius of curvature of a phase boundary appears on [https://books.google.com/books?id=xTYpAQAAIAAJ&pg=PA503#v=onepage&q&f=false page 503].
5. ^Freundlich, Herbert, Kapillarchemie: Eine Darstellung der Chemie der Kolloide und verwandter Gebiete [Capillary Chemistry: A presentation of colloid chemistry and related fields] (Leipzig, Germany: Akademische Verlagsgesellschaft, 1909), [https://books.google.com/books?id=HKWEAAAAIAAJ&pg=PA144#v=onepage&q&f=false page 144].
6. ^Sir William Thomson (1871) [https://books.google.com/books?id=ZeYXAAAAYAAJ&pg=PA448#v=onepage&q&f=false "On the equilibrium of vapour at a curved surface of liquid,"] Philosophical Magazine, series 4, 42 (282) : 448-452. See equation (2) on page 450.
7. ^The derivation here is based on pages 524-525 of: Robert von Helmholtz (1886) [https://books.google.com/books?id=9xVbAAAAYAAJ&pg=PA508#v=onepage&q&f=false "Untersuchungen über Dämpfe und Nebel, besonders über solche von Lösungen"] (Investigations of vapor and mists, and especially of such things from solutions), Annalen der Physik, 263 (4) : 508-543.
See also
- Köhler theory
- Kelvin equation
- Flat-footedness
- Flat Foot Four
- Flatfoot in Hong Kong
- Flat Foot Stooges
- Flatford: Bridge Cottage
- Flatford Mill
- Flat four
- Flat furniture
- Flat (geometry)
- Flatground ollie
- Flat-haired Mouse
- Flat-haired mouse
- Flat-head
- Flat Head
- Flat-head adder
- Flathead catshark
- Flat-headed adder
- Flat-headed Cusimanse
- Flat-headed frog
- Flat-headed Galaxias
- Flat-Headed Galaxias
- Flat-headed kusimanse
- Flat-headed mayfly
- Flat headed mayfly
- Flat-Headed Myotis