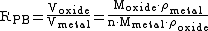- Definition
- History
- Application
- Values
- References
The Pilling–Bedworth ratio (P–B ratio), in corrosion of metals, is the ratio of the volume of the elementary cell of a metal oxide to the volume of the elementary cell of the corresponding metal (from which the oxide is created).
On the basis of the P–B ratio, it can be judged if the metal is likely to passivate in dry air by creation of a protective oxide layer.
Definition
where:
-
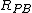 – Pilling–Bedworth ratio
– Pilling–Bedworth ratio -
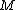 – atomic or molecular mass
– atomic or molecular mass -
 – number of atoms of metal per molecule of the oxide
– number of atoms of metal per molecule of the oxide -
 – density
– density -
 – molar volume
– molar volume
History
N.B. Pilling and R.E. Bedworth[1] suggested in 1923 that metals can be classed into two categories: those that form protective oxides, and those that cannot. They ascribed the protectiveness of the oxide to the volume the oxide takes in comparison to the volume of the metal used to produce this oxide in a corrosion process in dry air. The oxide layer would be unprotective if the ratio is less than unity because the film that forms on the metal surface is porous and/or cracked. Conversely, the metals with the ratio higher than 1 tend to be protective because they form an effective barrier that prevents the gas from further oxidizing the metal.[2]
Application
On the basis of measurements, the following connection can be shown:
- RPB < 1: the oxide coating layer is too thin, likely broken and provides no protective effect (for example magnesium)
- RPB > 2: the oxide coating chips off and provides no protective effect (example iron)
- 1 < RPB < 2: the oxide coating is passivating and provides a protecting effect against further surface oxidation (examples aluminium, titanium, chromium-containing steels).
However, the exceptions to the above P-B ratio rules are numerous. Many of the exceptions can be attributed to the mechanism of the oxide growth: the underlying assumption in the P-B ratio is that oxygen needs to diffuse through the oxide layer to the metal surface; in reality, it is often the metal ion that diffuses to the air-oxide interface. {{citation needed|date=February 2015}}
Values
| Metal | Metal oxide | Formula | RPB |
|---|---|---|---|
| Potassium | Potassium oxide | 2}}{{Oxygen}} | 0.474[4] |
| Sodium | Sodium oxide | 2}}{{Oxygen}} | 0.541[4] |
| Lithium | Lithium oxide | 2}}{{Oxygen}} | 0.567[4] |
| Strontium | Strontium oxide | {{Strontium}}{{Oxygen}} | 0.611[4] |
| Calcium | Calcium oxide | {{Calcium}}{{Oxygen}} | 0.64 [2] |
| Barium | Barium oxide | {{Barium}}{{Oxygen}} | 0.67[4] |
| Magnesium | Magnesium oxide | {{Magnesium}}{{Oxygen}} | 0.81 |
| Aluminium | Aluminium oxide | 2}}{{Oxygen|3}} | 1.28 |
| Lead | Lead(II) oxide | {{Plumbum}}{{Oxygen}} | 1.28 [2] |
| Platinum | Platinum(II) oxide | {{Platinum}}{{Oxygen}} | 1.56 [2] |
| Zirconium | Zirconium(IV) oxide | 2}} | 1.56 |
| Zinc | Zinc oxide | {{Zinc}}{{Oxygen}} | 1.58 |
| Hafnium | Hafnium(IV) oxide | 2}} | 1.62 [2] |
| Nickel | Nickel(II) oxide | {{Nickel}}{{Oxygen}} | 1.65 |
| Iron | Iron(II) oxide | {{Iron}}{{Oxygen}} | 1.7 |
| Titanium | Titanium(IV) oxide | 2}} | 1.73 |
| Iron | Iron(II,III) oxide | 3}}{{Oxygen|4}} | 1.90 |
| Chromium | Chromium(III) oxide | 2}}{{Oxygen|3}} | 2.07 |
| Iron | Iron(III) oxide | 2}}{{Oxygen|3}} | 2.14 |
| Silicon | Silicon dioxide | 2}} | 2.15 |
| Tantalum | Tantalum(V) oxide | 2}}{{Oxygen|5}} | 2.47 [2] |
| Niobium | Niobium pentoxide | 2}}{{Oxygen|5}} | 2.69[4] |
| Vanadium | Vanadium(V) oxide | 2}}{{Oxygen|5}} | 3.25 [2] |
References
2. ^1 2 3 4 5 6 "ASM Handbook Vol.13 Corrosion", ASM International, 1987
3. ^1 2 3 4 5 6 {{Cite web |title=Pilling–Bedworth ratios (PBRs) for metals and their oxides |url=http://pubs.acs.org/subscribe/archive/ci/31/i11/html/11sheppard_tab1.ci.html}}
}}{{DEFAULTSORT:Pilling-Bedworth ratio}}
- Emperor Yizong of Western Xia
- Emperor Yomei of Japan
- Emperor Yongle
- Emperor Yongle of China
- Emperor Yongzheng
- Emperor Yozei of Japan
- Emperor Yuan of Han
- Emperor Yuan of Han China
- Emperor Yuan of Jin
- Emperor Yuan of Wei China
- Emperor Yuryaku of Japan
- Emperor Yōmei
- Emperor Yōzei
- Emperor Yōzei of Japan
- Emperor Yūryaku
- Emperor Zeno
- Emperor Zhang of Han
- Emperor Zhao of Han
- Emperor Zhao of Han China
- Emperor Zhaozong
- Emperor Zhenzong of Song China
- Emperor Zhezong
- Emperor Zhezong of Song China
- Emperor Zhi of Han
- Emperor Zhongzong of Tang