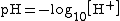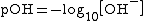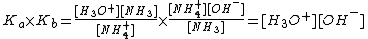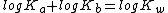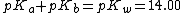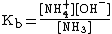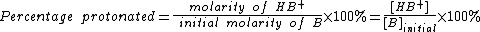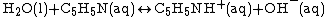- pH, Kb, and Kw
- Percentage protonated
- A typical pH problem
- Examples
- Simple Facts
- See also
- References
- External links
On dissolving in water, a weak base does not dissociate completely and the resulting aqueous solution contains OH- ion and the concerned basic radical in a small proportion along with a large proportion of undissociated molecules of the base.
pH, Kb, and Kw
Bases range from a pH of greater than 7 (7 is neutral, like pure water) to 14 (though some bases are greater than 14). pH has the formula:
Since bases are proton acceptors, the base receives a hydrogen ion from water, H2O, and the remaining H+ concentration in the solution determines pH. Weak bases will have a higher H+ concentration because they are less completely protonated than stronger bases and, therefore, more hydrogen ions remain in the solution. If you plug in a higher H+ concentration into the formula, a low pH results. However, pH of bases is usually calculated using the OH− concentration to find the pOH first. This is done because the H+ concentration is not a part of the reaction, while the OH− concentration is.
By multiplying a conjugate acid (such as NH4+) and a conjugate base (such as NH3) the following is given:
Since 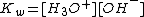 then,
then, 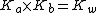
By taking logarithms of both sides of the equation, the following is reached:
Finally, multiplying throughout the equation by -1, the equation turns into:
After acquiring pOH from the previous pOH formula, pH can be calculated using the formula pH = pKw - pOH where pKw = 14.00.
Weak bases exist in chemical equilibrium much in the same way as weak acids do, with a base dissociation constant (Kb) indicating the strength of the base. For example, when ammonia is put in water, the following equilibrium is set up:
Bases that have a large Kb will ionize more completely and are thus stronger bases. As stated above, pH of the solution depends on the H+ concentration, which is related to the OH− concentration by the self-ionization constant (Kw = 1.0x10−14). A strong base has a lower H+ concentration because they are fully protonated and less hydrogen ions remain in the solution. A lower H+ concentration also means a higher OH− concentration and therefore, a larger Kb.
NaOH (s) (sodium hydroxide) is a stronger base than (CH3CH2)2NH (l) (diethylamine) which is a stronger base than NH3 (g) (ammonia). As the bases get weaker, the smaller the Kb values become.[1]
Percentage protonated
As seen above, the strength of a base depends primarily on pH. To help describe the strengths of weak bases, it is helpful to know the percentage protonated-the percentage of base molecules that have been protonated. A lower percentage will correspond with a lower pH because both numbers result from the amount of protonation. A weak base is less protonated, leading to a lower pH and a lower percentage protonated.[2]
The typical proton transfer equilibrium appears as such:
B represents the base.
In this formula, [B]initial is the initial molar concentration of the base, assuming that no protonation has occurred.
A typical pH problem
Calculate the pH and percentage protonation of a .20 M aqueous solution of pyridine, C5H5N. The Kb for C5H5N is 1.8 x 10−9.[3]
First, write the proton transfer equilibrium:
The equilibrium table, with all concentrations in moles per liter, is
| C5H5N | C5H6N+ | OH− | |
|---|---|---|---|
| initial normality | .20 | 0 | 0 |
| change in normality | -x | +x | +x |
| equilibrium normality | .20 -x | x | x |
| Substitute the equilibrium molarities into the basicity constant | 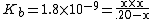 |
| We can assume that x is so small that it will be meaningless by the time we use significant figures. | 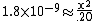 |
| Solve for x. | 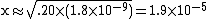 |
| Check the assumption that x << .20 | 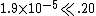 ; so the approximation is valid ; so the approximation is valid |
| Find pOH from pOH = -log [OH−] with [OH−]=x | 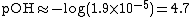 |
| From pH = pKw - pOH, | 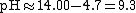 |
| From the equation for percentage protonated with [HB+] = x and [B]initial = .20, | 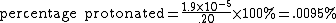 |
This means .0095% of the pyridine is in the protonated form of C5H5NH+.
Examples
- Alanine
- Ammonia, NH3
- Methylamine, CH3NH2
- Ammoniumhydroxide, NH4OH
Simple Facts
- An example of a weak base is ammonia. It does not contain hydroxide ions, but it reacts with water to produce ammonium ions and hydroxide ions.[4]
- The position of equilibrium varies from base to base when a weak base reacts with water. The further to the left it is, the weaker the base.[5]
- When there is a hydrogen ion gradient between two sides of the biological membrane, the concentration of some weak bases are focused on only one side of the membrane.[6] Weak bases tend to build up in acidic fluids.[6] Acid gastric contains a higher concentration of weak base than plasma.[6] Acid urine, compared to alkaline urine, excretes weak bases at a faster rate.[6]
See also
- Strong base
- Weak acid
References
2. ^{{cite book|author=Howard Maskill|title=The physical basis of organic chemistry|url=https://books.google.com/books?id=4AXwAAAAMAAJ|year=1985|publisher=Oxford University Press, Incorporated|isbn=978-0-19-855192-8}}
3. ^{{cite web|url=http://www.kentchemistry.com/links/AcidsBases/pHWeakBases.htm|title=Calculations of weak bases|publisher=Mr Kent's Chemistry Page|accessdate=2018-03-23}}
4. ^Atkins, Peter, and Loretta Jones. Chemical Principles: The Quest for Insight, 3rd Ed., New York: W.H. Freeman, 2005.
5. ^Clark, Jim. "Strong and Weak Bases."N.p.,2002. Web.
6. ^1 2 3 {{cite web|last1=Milne|last2=Scribner|last3=Crawford|title=Non-ionic Diffusion and the Excretion of Weak Acids and Bases|url=http://ac.els-cdn.com/0002934358903760/1-s2.0-0002934358903760-main.pdf?_tid=cf6217f0-b7e1-11e4-97d9-00000aacb360&acdnat=1424314300_581ecc26daaa9efc9c9af94344e96ef2|website=Science Direct|accessdate=19 February 2015}}
External links
- Guide to Weak Bases from Georgetown course notes
- [https://web.archive.org/web/20070926225948/http://www.intute.ac.uk/sciences/reference/plambeck/chem1/p01154.htm Article on Acidity of Solutions of Weak Bases] from Intute
- Freeform channel
- Free-form construction
- Freeform curve
- Free Form Fabrication
- Freeform Go
- Free form (linguistics)
- Free Form Patterns
- Freeform Portland
- Freeform (radio format)
- Freeform's 25 Days of Christmas
- Freeform (TV channel)
- Freeform (TV network)
- Freeform Worldwide
- Freeform Worldwide Inc.
- Free-free emission
- Free Free Free
- Free French Air Force
- Free French Camel Corps
- Free French partisans
- Free Fringe (disambiguation)
- Freeganj
- FREE (genre)
- Free Germania
- Free-Germany Movement
- Free Germany Movement