燃料酒精fuel alcohol
亦称燃料乙醇。以甘蔗、甜高粱秆、甜菜、木薯、土豆等农作物为原料制取。常用的农作物原料及乙醇的平均产量见表1。
表1 用于制取乙醇的原料及产量
| 作物名称 | 作物产量 (吨/公顷·年) | 乙 醇 产 量 | |
| (升/吨) | (升/公顷·年) | ||
| 甘 蔗 甜高粱秆 甜 菜 饲料甜菜 小 麦 大 麦 玉 米 高 粱 木 薯 甘 薯 土 豆 | 50~90 45~80 15~50 100~200 1.5~2.1 1.2~2.5 1.7~5.4 1.0~3.7 10~65 8~50 10~25 | 70~90 60~80 90 90 340 250 360 350 170 167 110 | 3 500~8 000 1 750~5 300 1 350~5 500 4 400~9 350 510~714 300~625 600~1 949 350~1 295 1 700~11 050 1 336~8 350 1 110~2 750 |
注: 每生产1吨糖,大致可得糖蜜300千克,每吨糖蜜可制乙醇245升。
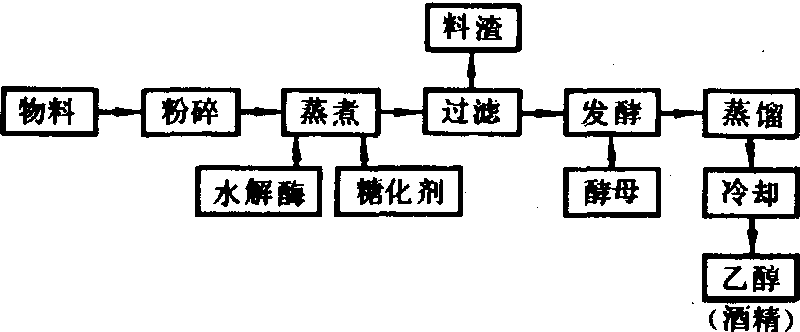
通常用发酵法制取乙醇,其工艺流程如下图所示:
酒精对水有较强的亲和性,容易不断吸收空气中的水分,从而降低其热值和汽化性能。酒精、汽油的物性对比见表2。
表2 酒精与汽油的物性对比
| 名 称 | 乙 醇 | 汽 油 |
| 密度(克/厘米3) 沸点(℃) 比热(千焦/千克度) 气化潜热(千焦/千克) 辛烷值 热值(千焦/千克) | 0.789 78.5 2.29 855 100 26 832 | 0.7~0.75 30~200 2.43 272 76 40 248 |
酒精作为燃料,主要掺入汽油中使用。酒精是一种溶剂,对一些橡胶、塑料零件会产生不良影响。酒精的热值仅是汽油的1/2~2/3,同一功率下的消耗量比汽油多。单独使用时,汽化器须进行改装。酒精含有一定量氧气,空燃比为9:1(汽油为1.49:1),所以目前单独使用酒精尚难以和汽油竞争,但酒精的辛烷值高,抗爆性好,掺入汽油中使用能大大改善发动机的抗爆性,使发动机噪音小,运转平稳。用乙醇代替常用有毒性的抗爆剂四乙铅,能减少废气中的CO、NOx和HC的含量,从而减轻对大气的污染。在掺入使用中,当酒精中含有水分比例较大时,酒精、汽油混合液会出现分层,使发动机燃烧不稳定。所以一般掺入含水量不超过1.5%的酒精,混合后的燃料中酒精含量不超过10%,对发动机的动力性、经济性影响不大。若采用两个供油系统,可避免这一现象。
酒精的十六烷值很低,约为8,故不宜作为柴油机的代用燃料。