老虎冲遗址
青铜时代遗址。位于沈阳市苏家屯区陈相屯镇沈阳市储运公司砖厂南部,1978年和1979年两次试掘,面积约1000平方米。其遗迹有16个用白膏泥填充的坑,由南至北一线排列。坑有圆形和长圆形,直径大小不一,大者2米,小者1米,中者1. 5米,深0.35~1. 6米不等。坑壁为夯土,用白泥和砂土夯筑,每层厚5厘米,坑内用白膏泥填充。坑边出有树叶、树枝、桦树皮、榛子皮、布痕、木碳等。坑内用木头拌子搭成井字形架,出土有黑色素面陶器,器型有高领罐、双耳壶、罐式鼎、深裆鬲、纺轮、木镰,采集有石斧、石刀等。据推测应是古代的祭祀坑,距今约3000年左右。
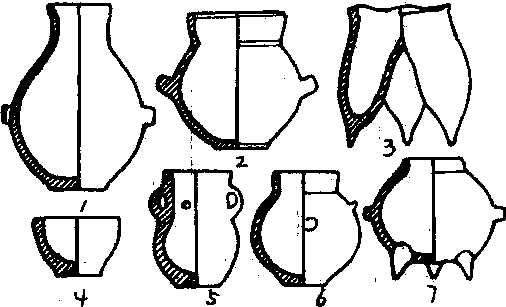
(1.5陶壶2. 6陶罐 3. 陶鬲 4. 陶杯. 7. 陶鼎)
老虎冲遗址陶器